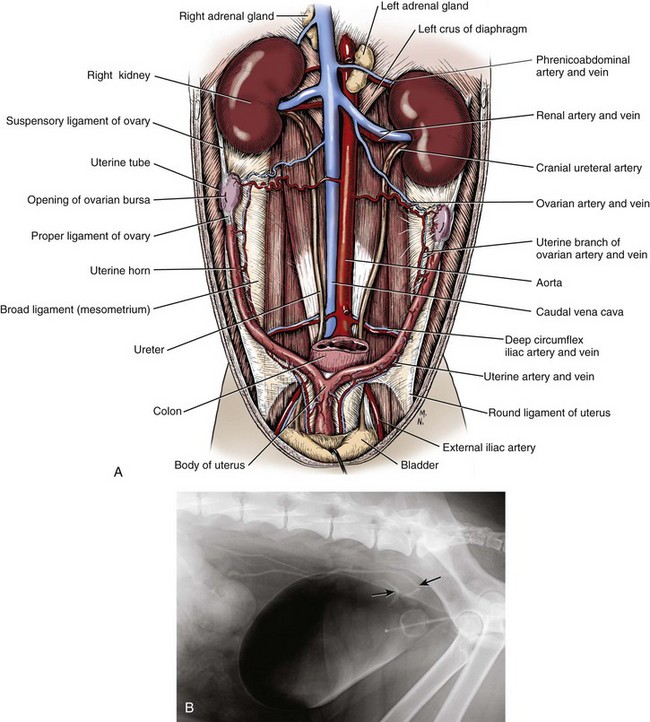
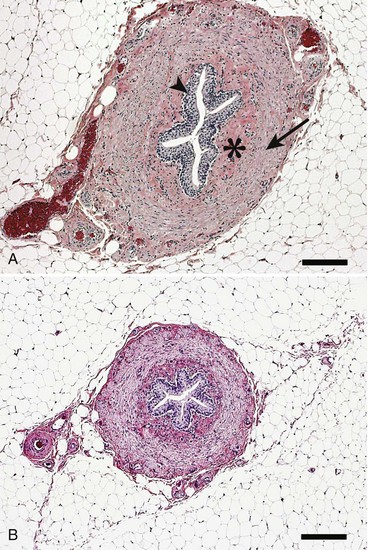
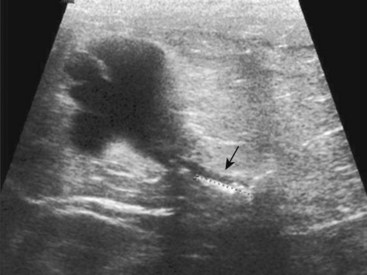
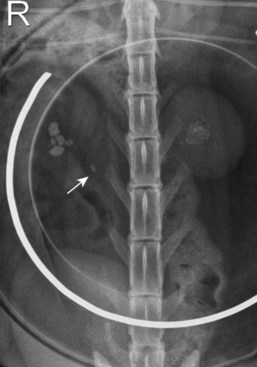
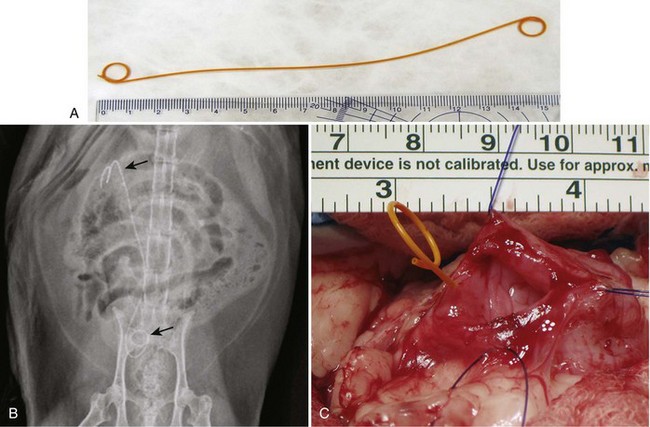
Chapter 115 The ureters are paired fibromuscular tubes that transport urine from the renal pelves to the urinary bladder via peristaltic activity. The length and diameter of normal canine and feline ureters are poorly documented but vary among species and breeds. The luminal diameter of a nonobstructed distal feline ureter has been anecdotally reported to be approximately 0.4 mm, and 2.5-Fr (0.8-mm) stents are now being placed as treatment for obstructive feline ureterolithiasis at some institutions.12,15,80 Similarly, the normal diameter of the canine ureter likely varies depending on the size of the dog, although on ventrodorsal excretory urography, a general guideline of 0.07 times the length of the body of the second lumbar vertebra has been used as the normal luminal diameter of the upper canine ureter.48 One study that evaluated movement of steel spheres from the proximal canine ureter in dogs weighing 9 to 23 kg noted that it was difficult to insert spheres 3.9 mm in diameter, but 2.3-mm spheres moved easily within the ureteral lumina.79 Computed tomographic (CT) measurement of the ureteral diameter averaged 2.0 to 2.5 mm before and after contrast administration in six dogs weighing 21 to 30 kg.129 After leaving the renal pelvis dorsolateral to the renal vessels, the ureters course ventral to the psoas major and minor muscles in the retroperitoneal space (Figure 115-1).45 The right ureter lies just lateral to the caudal vena cava and occasionally may pass dorsal to the vena cava before returning to its typical course.31,39 After passing ventral to the external iliac vessels, the ureters turn ventrally toward the trigone of the urinary bladder. In males, the ureters course dorsal to the ductus deferens before entering the wall of the bladder. Just cranial to their vesicular attachment, they recurve slightly, resulting in a “J shape” that has been described as a normal finding on ureterographic studies (see Figure 115-1, B).28,47 The ureters then run obliquely within the wall of the bladder for a short distance toward the trigone before emptying into the lumen through slitlike or “horseshoe-shaped” orifices cranial to the internal urethral sphincter.28 The intramural portion of the ureter crosses and is variably attached to the outer longitudinal, middle circular, and inner longitudinal layers of the detrusor muscle, becoming submucosal just proximal to the ureteral orifice.28 The ureteral blood supply originates cranially and caudally. The ureteral artery, arising from the caudal aspect of the renal artery, runs caudally along the adventitial surface of the ureter and anastomoses with the ureteric branch of the caudal vesicular artery, which in turn arises ultimately from either the prostatic or vaginal artery.45 Autonomic nerves to the ureter arise from the celiac and pelvic plexuses. The ureters are composed of three tissue layers, including an outer adventitial layer, a central muscular layer, and an inner mucosal layer (Figure 115-2).35,42,154 Although there is limited information available on the individual layers in cats and dogs, the muscular layer in dogs accounts for approximately 50% of ureteral wall thickness (if adventitia is not included), and the transitional epithelium and underlying lamina propria of mucosal layer make up approximately 15% and 30%, respectively.159 The adventitia is composed of fibroelastic connective tissue.42,132,154 Vessels, nerves, and lymphatics within the different ureteral layers are intimately associated with one another, forming a plexus within the adventitia that communicates with a second plexus running along the outer surface of the tunica muscularis.42,132 Innervation of the ureter has been shown to have both sympathetic and parasympathetic components.42 Vessels and associated nerves penetrate the muscularis to the level of the lamina propria, where they form another plexus before sending branches to the epithelium.42,132 The tunica muscularis is commonly subdivided into three layers on cross-section, including an inner and outer longitudinal layer and a middle circular layer. However, the fiber pitch of the middle layer varies from circular to oblique, and if traced, the fibers originate and end within the longitudinal layers.8 The pitch of the muscle fibers in the canine ureter is more circular proximally, becomes more oblique toward midlength, and then is almost longitudinal distally.8 Although the canine tunica muscularis comprises half or more of the cross-sectional area, some have found the muscularis to be thinner near the renal pelvis.132 The mucosal layer is subdivided into lamina propria and transitional epithelial layers.35,154,159 The lamina propria, which lies between the muscularis and the epithelium, is composed of collagen fibers, fibrocytes, interlacing blood and lymphatic vessels, and unmyelinated nerve fibers. It is approximately one quarter to one half the thickness of the tunica muscularis. The transitional epithelium is typically four to six cell layers in thickness and is thrown into longitudinal folds when the ureteral lumen is not distended. The layer adjacent to the lumen consists of large polygonal cells. The cells in the next two or three layers vary in size and shape, and in the deepest layer, the cells are cuboidal to columnar. In the human ureter, all of these cells have been shown to have microvilli on all surfaces except for the deep surface of the basal layer adjacent to the basement membrane. The microvilli projecting into the lumen are surrounded by a mucinous coat. Those between cell layers protrude into the extracellular space, which contains a “slimy cement substance” that may allow these cells to slide past one another during luminal dilatation.154 The series of physiologic events that occurs after ureteral obstruction is complex, and after relief of the obstruction, changes continue to occur in the previously obstructed kidney. Most studies have looked at canine, porcine, and rat models, and there are numerous species differences.56,94,112 In addition, the duration and degree of occlusion affect the kidney’s response to and recovery from obstruction. In general, after unilateral obstruction, ureteral pressures increase and peak by 5 hours and then lessen but remain increased 12 to 24 hours after obstruction.156 After a transient increase, renal blood flow diminishes to 40% of normal over the first 24 hours and continues to decrease to 20% of normal by 2 weeks. The increase in pressure, which is transmitted to the renal tubules and glomeruli, and decrease in blood flow are accompanied by a decrease in glomerular filtration rate through a complex interaction of a variety of vasoactive mediators.156,158 There is a compensatory increase in glomerular filtration rate in the contralateral kidney. A leukocyte influx (macrophages and T-lymphocytes) into the obstructed kidney also occurs. Macrophages release proteolytic enzymes and cytokines that may result in fibroblast recruitment and activation and have negative effects on renal blood flow.156 Activated fibroblasts may, in turn, contribute to development of interstitial fibrosis or glomerulosclerosis. The longer the duration of ureteral obstruction, the less likely that the kidney will recover to where the animal is no longer azotemic. In normal dogs, after 1 week of obstruction, glomerular filtration rate returns to 65% of control values after the ureter is unobstructed. Maximum recovery occurs by approximately 5 weeks.50 After 2 weeks of obstruction, glomerular filtration rate returns to only 46% of normal over 4 months.78,153,156 Slight, moderate, and severe tubular dilatation with interstitial fibrosis occur after 1, 2, and 3 weeks of obstruction, respectively.50 Given that these were normal dogs, azotemia after renal recovery would not be expected; no definitive prognostications can be made regarding dogs or cats with bilateral renal disease and ureteral obstruction secondary to ureterolithiasis. Changes have also been shown to occur in the obstructed ureter itself. Rat ureteral smooth muscle hypertrophies after obstruction and is then gradually replaced with fibrous tissue over roughly 6 weeks.29 Again, whether the same changes occur over a similar time course in dogs and cats is conjectural. If a ureterolith is present, many clinicians attempt to diurese the patient for some amount of time (1 to 4 days) before surgery, and some have attempted to induce relaxation of ureteral smooth muscle to get the ureterolith to move into the urinary bladder.* This would eliminate the need for a ureterotomy, which is more difficult than a cystotomy. Drugs that have been shown to induce ureteral relaxation in other species (e.g., calcium channel blockers, glucagon, amitriptyline) have not been widely investigated for treatment of feline or canine ureterolithiasis.1 In 18 cats with ureterolithiasis, administration of glucagon (0.05 to 0.1 mg/cat IV 1 to 12 times over a 72-hour period) resulted in stone movement in only four cases and was associated with several side effects.52 Use of the tricyclic antidepressant amitriptyline in cats may have some beneficial effect when treating acute obstructions (10 mg/cat PO q24hr BID while on fluids); in one study, four of four stones passed within 7 days.93 Significant inflammation and fibrosis of the ureter adjacent to the stone are associated with a poor response (zero of five stones moved).93 The benefits of potentially avoiding surgery must be weighed against the risks of increased renal damage secondary to prolonged obstruction. The degree of obstruction (partial versus complete) also needs to be taken into consideration. In one study of 153 cats, 52 cats were treated solely by medical management; 9 of 14 that had serial imaging performed had migration of a ureterolith into the bladder.86 An additional 101 cats in the study required surgery for ureteroliths that did not migrate with medical management.86 The average wait from start of medical management to surgery decreased from 4 days before the year 2000 to 2 days after the year 2000. Of the 52 cats in which medical management alone was attempted, 17 were euthanized or died, 12 were lost to follow-up, 16 had no change in creatinine, and only seven had a significant improvement in creatinine with medical management alone. One- and 2-year survival statistics were 66% and 66%, respectively, compared with 91% and 88% for those treated surgically.86 Extracorporeal shockwave lithotripsy to fragment feline ureterolithiasis has not been widely used because it may result in renal injury when applied to concomitant nephroliths.3 Decreasing shockwave doses in an attempt to avoid renal injury has resulted in a lack of efficacy.3,90 The safety and efficacy of lithotriptors that are better able to focus on feline ureteroliths requires further study.88,90 Several important points must be made to owners before considering surgery to correct feline ureterolithiasis. First, every cat has a different amount of damage to the obstructed kidney, and the clinician cannot predict how long the obstruction has been present or how well the cat will rebound; in other words, the clinician cannot tell if the cat’s creatinine will decrease only slightly or completely normalize. In one report, whereas 2 of 11 cats with marked azotemia improved but remained azotemic postoperatively, 8 of 8 with mild azotemia (creatinine, 2.1 to 3.1 mg/dL) remained azotemic 12 to 30 months later.87 Second, results of kidney biopsies from cats that have undergone ureteral surgery secondary to obstruction from calcium oxalate ureteroliths have shown that most have some degree of chronic interstitial nephritis (personal experience). This is a progressive condition that may eventually result in renal function deterioration, despite surgery. Because these biopsies came from the ipsilateral kidney, it is possible that the inflammation noted may have been secondary to the obstruction itself.29,32,156 Further study focusing on the pathologic changes in the contralateral unobstructed kidney is needed. Third, if an azotemic cat has unilateral ureteral obstruction, it must have bilateral renal disease; otherwise, the cat would not be azotemic or uremic. In one study, 58 of 76 cats with unilateral calculi were azotemic, and 39 of 70 contralateral kidneys were small when evaluated by ultrasonography before surgery for unilateral ureterolithiasis.85 In another study, relative glomerular filtration rate was calculated for 3 of 11 cats before surgery.87 The obstructed kidney contributed 75% or more of the total glomerular filtration rate in two of these three cats. Fixing the obstruction does not take care of the underlying renal disease. The obstruction likely “pushed the cat over the edge” (i.e., exacerbated an underlying condition [acute on chronic renal failure]), resulting in azotemia and uremic clinical signs. And finally, significant complications occurred in one third of feline cases that were taken to surgery to remove ureterolith(s), with overall mortality rate in one study reported to be 18%.86 The most common complication was development of uroabdomen (16% of cases).86 Uroabdomen may occur secondary to leakage at the ureterotomy or ureteral reimplantation site or from the greater curvature of the kidney at the nephrostomy tube site. Newer nephrostomy tubes designed for percutaneous placement may result in less risk of leakage and have been used successfully in the pre- and postoperative periods for renal decompression.12,16 Localization of the ureterolith(s) is most commonly performed using abdominal ultrasonography (Figure 115-3). Although many calcium oxalate ureteral calculi (the most common mineral type in cats) can be seen on plain radiographs (Figure 115-4), small or radiolucent calculi can be missed. Ultrasonography has the added benefit of allowing the clinician to determine the degree of ureteral and renal pelvic dilatation present. If mineralized calculi are not evident on ultrasonographic examination, ureteral dilatation or hydronephrosis may be secondary to a ureteral stricture from a previous calculus or a blood calculus may be present. Hematuria appears to be commonly seen on urinalysis of cats with obstructive blood calculi.157 Excretory urography can also be performed, but image quality may suffer in animals that are azotemic, and there is some risk for inducing further renal damage if the patient is not adequately hydrated. After the animal has been stabilized and it has been determined that diuresis has failed to move the calculus, a ureteral resection or reimplantation or, less commonly, ureterotomy is performed (see techniques section below). More recently, placement of double-pigtail ureteral catheters under fluoroscopic or ultrasound guidance has gained favor.12,15 In most cases, a ureterotomy is not performed, thus eliminating the risk of postoperative leakage at this site. Placement of a feeding tube should be considered before abdominal closure if the cat has been anorectic. One should consider placing an abdominal drain (e.g., multifenestrated, silicone, continuous suction drain) before closure of the linea so that urine leakage may be easily assessed. Ureteral Catheter Placement.: Although catheter placement procedure can be performed with the aid of a cystoscope (nonsurgical), this is less successful than surgical placement (30% versus 82.4%) through a ventral midline celiotomy and cystotomy.15 A guidewire is placed, either retrograde from the ureteral orifice to the renal pelvis or normograde through the greater curvature of the kidney, down the ureter, past the ureterolith, and into the bladder. A ureteral dilator is then passed over this wire, followed by placement of a double-pigtail indwelling catheter (Figure 115-5). One end of the catheter lies within the renal pelvis and the other in the bladder lumen. The ureterolith(s) are not removed. Reported complications include temporary stranguria (5 of 18 cats), imperfect stent location (2 of 18 cats), ureteral trauma (1 of 18 cats), and urinary tract infection that cleared with antibiotic therapy (4 of 16 cats).15 Rarely, proximally located ureteroliths and nephroliths have been removed with the aid of an endoscope placed through the greater curvature of the kidney and into the renal pelvis either surgically or percutaneously.12,82 Urine leakage is the most common postoperative complication and was reported in 8 of 64 (12%) of cats after the use of nephrostomy tubes was abandoned.86 Ureterotomy leakage is common after ureterolithotomy in humans, and the abdominal drain is left in place until the ureter heals and leakage stops.4 In dogs, ureterotomies that are left open will heal within 12 days if urine is evacuated from the abdomen;100 however, a similar study has not been reported in cats. In one report, two of five cats with uroabdomen after ureterotomy had urine drained via nephrostomy tubes (not abdominal drains) and went on to heal without further surgery.87 To date, waiting to see if uroabdomen will resolve in cats with abdominal drains has not been reported, but it may make sense as long as the animal is not deteriorating clinically and the creatinine is not rising. However, fibrosis and partial ureteral obstruction were shown to be more common in pigs if the ureter was allowed to heal by second intention.160 Ureteral obstruction secondary to urolithiasis is much less commonly reported in dogs.* Out of 11,000 canine uroliths submitted over a 13-year period to one laboratory, only 61 (1.1% of specimens) were ureteroliths; 32 of the 34 ureteroliths that were submitted for aerobic culture grew bacteria, with Staphylococcus spp. and Escherichia coli being the most common isolates.95 Approximately half of the dogs with ureteroliths had concomitant nephroliths or cystoliths.96 Although mineral composition, breed, and gender associated with renal calculi in dogs have been reported, few evaluations have focused on canine ureteral calculi.95,96,128,140 In one report of 16 dogs surgically treated for ureterolithiasis, females were overrepresented.140 The mean age was 8 years, and the median body weight was 9.7 kg. Ureterolith composition was most commonly either struvite (n = 6) or calcium oxalate (n = 5). Struvite calculi were associated with increased peripheral white blood cell count and purulent discharge at the surgery site. All cases with purulent discharge had positive aerobic culture results. Although hydroureter and hydronephrosis were present proximal to all ureteroliths, only eight dogs had an increased serum creatinine concentration before surgery. The most common historical findings were lethargy, vomiting, and anorexia. Thirteen dogs had abdominal pain on palpation, seven had pyrexia, 12 had hematuria, and nine had pyuria. All dogs recovered well from surgery, but one was reoperated twice for recurrent nephroliths and ureteroliths. A second dog was taken back to surgery after development of a ureteral stricture.140 Guidelines and techniques for treatment of canine ureteroliths appear to be similar to those for feline ureteroliths. Similar to cats, surgical removal can be challenging in small-breed dogs. In larger dogs, techniques such as ureterotomy or neoureterocystostomy are easily performed, often without magnification. The use of double-pigtail ureteral stents to allow for urine flow around obstructive ureteroliths has also been recently reported in dogs.114 Lithotripsy.: As an alternative to surgical removal of canine ureteroliths, extracorporeal shockwave lithotripsy has been used to treat select cases, most having concomitant nephrolithiasis.7,20 Unfortunately, lithotriptors are only available at a small number of referral institutions. In humans, lithotripsy is considered the first line of treatment for proximal ureteroliths 1 cm or smaller in diameter.134 A combination of lithotripsy and ureteroscopy is typically used for similarly sized stones in the lower ureter. Ureteroliths smaller than 5 mm in diameter usually pass spontaneously in human beings.134 Similar lithotripsy guidelines for canine ureterolithiasis are not available. In the literature, dogs treated with lithotripsy have had ureteral obstruction, refractory pyelonephritis with nephroliths and ureteroliths, or progressive chronic renal failure with concurrent nephrolith development.7,20 Success rates in dogs likely depends on the presence of concomitant nephroliths, ureterolith composition and relative size compared with ureteral diameter, type of lithotriptor used, and dose and frequency of shockwaves used.3,90 Compared with nephroliths, ureteroliths are typically more difficult to fragment.3 In one study, successful treatment was reported in six of seven dogs with ureteroliths, although four of seven dogs required more than one retreatment.3 All dogs had concurrent nephrolithiasis. An update by the same author indicated that 20 of 25 dogs with ureteroliths were successfully treated with lithotripsy.2 Again, approximately half of these cases required more than one treatment. Primary ureteral neoplasia is quite rare in dogs and has not been reported in cats (Table 115-1). Secondary involvement of a ureter by tumors of the urinary bladder or a kidney is more commonly reported. Treatment of urinary bladder tumors may include ureteral reimplantation or less commonly performed procedures, such as ureterocolonic anastomosis (see Chapters 114 and 116 for treatment of renal and bladder tumors). Ureteronephrectomy is usually performed when primary ureteral tumors do occur, although ureteral reimplantation or resection and anastomosis can be performed in select cases if clean surgical margins are obtainable. Table • 115-1 Summary of Small Animal Ureteral Tumors Reported in the Veterinary Literature R&A, Resection and anastomosis; UN, ureteronephrectomy; UTI, urinary tract infection. Animals with ureteral masses may present with hematuria, polyuria, polydipsia, anorexia, lethargy, pyrexia, or abdominal pain possibly from renal capsular stretching associated with obstructive hydronephrosis. Benign fibroepithelial polyps make up 7 of 15 primary canine ureteral tumors in the literature, in contrast to humans, in whom transitional cell carcinoma is most common.49 Fibroepithelial polyps have also been reported in humans; they occur more commonly in men and tend to develop in the proximal ureter. There have not been enough canine cases to make a similar assessment. Extremely rare conditions, such as granulomatous ureteritis in a dog and retroperitoneal thrombosis with secondary periureteral fat necrosis in a cat, have been reported and should be considered as differential diagnoses for ureteral masses.33,125 Ureteral trauma is uncommonly reported and, when it occurs, is most often iatrogenic and associated with ovariohysterectomy. This is similar to the situation in humans, in whom the majority of ureteral injuries are incurred during gynecologic surgery.43,57 The true incidence of ureteral injury associated with ovariohysterectomy is not known because studies that have evaluated spay-related complications have not performed routine abdominal imaging.17,23,38,121 Ovariohysterectomy-associated ureteral injury is more commonly reported in dogs than in cats and may be secondary to inadvertent ligation, transection, or resection or from obstruction secondary to ovarian, uterine, or vaginal granuloma formation.60,77,106,118,151 Factors such as surgeon experience or suture material used for ovariohysterectomy are generally not reported. Clinical signs associated with uremia (e.g., vomiting, lethargy) may occur shortly after injury if uroabdomen or bilateral ureteral obstruction occurs. Often, however, signs are not immediately apparent (e.g., hydronephrosis) if there is unilateral obstruction, partial bilateral obstruction, or unilateral retroperitoneal urine accumulation.* Ureterovaginal Fistula.: Rarely, ureterovaginal fistulas and associated urinary incontinence may develop.36,99,122 Ureterovaginal fistulae may occur when the encircling ligature placed around the uterine stump incorporates the distal ureter. It is unknown whether uterine stump granuloma with adhesions can result in fistula formation without the presence of an encircling ligature. The diagnosis is based on results of retrograde vaginography or excretory urogram, which indicate communication between the two structures. Treatment involves ureteral reimplantation, if possible, or ureteronephrectomy. In humans, ureteral tearing or avulsion is uncommon. It occurs primarily in children at the ureteropelvic junction and is secondary to rapid deceleration associated with automobile accidents or falls. Several veterinary cases of ureteral damage secondary to blunt abdominal trauma have been reported; there does not appear to be a site predilection in small animals.57,155 Blunt abdominal trauma may result in a ureteral crushing injury with subsequent obstruction and hydronephrosis.27 More commonly, however, uroabdomen or uroretroperitoneum occurs.64,113,135,155,161 Clinical signs associated with uroperitoneum include lethargy, vomiting, anorexia, abdominal pain, ascites, hypothermia, and possible hematuria.55 Affected animals may have mild to marked azotemia. Animals with uroretroperitoneum tend to have more subtle signs. They generally only have mild to moderate azotemia, exhibit pain on abdominal palpation, and may have hematuria.155 Hematuria is not a consistent finding in patients with external (blunt or penetrating) abdominal trauma with ureteral injury.43,57,105,124 Plain abdominal radiographs may indicate a loss of abdominal detail (uroabdomen) or retroperitoneal “streaking” with retroperitoneal urine accumulation. The diagnosis of uroabdomen or uroretroperitoneum is confirmed based on increased abdominal or retroperitoneal fluid creatinine and potassium concentrations compared with those in the serum. Excretory urography is used after the patient has been rehydrated and stabilized because intravenous contrast administered to dehydrated patients may result in further renal injury and may be difficult to interpret because of poor renal or ureteral opacification.55,105,124 CT pyelography and retrograde or antegrade pyelography may also be of benefit in identifying the site of injury if the patient is stable enough to undergo these procedures.43,57,105,119 Surgical exploration or examination of the ureters without preoperative localization of ureteral tears may be required in humans with penetrating abdominal injuries (gunshot or stab wounds) in whom surgery cannot be delayed.43 Similar penetrating injuries to the ureters in veterinary patients have apparently not been reported. Urinoma.: Uroretroperitoneum may result in the formation of a urinoma. A urinoma is a collection of urine within the retroperitoneum that causes fat necrosis and subsequent reactive fibrosis. The terms paraureteral and uriniferous pseudocyst have also been used to describe this condition and should not be confused with feline paranephric pseudocysts.116 The affected animal may present for a painful or nonpainful swelling in the sublumbar region several weeks after abdominal trauma. Mild to moderate azotemia may be present. Treatment includes ureteronephrectomy with ablation or omentalization of the urinoma cavity.6,113,152,161 Ureteral ectopia is a congenital anomaly of the distal ureter resulting in urinary incontinence. In dogs, the large majority of ectopic ureters are intramural; they enter the bladder wall in the normal anatomic location; however, rather than ending at the trigone of the bladder, they continue to travel submucosally to open within the urethra or vagina.71 In cats, and less commonly in dogs, ectopic ureters may run completely separate from the bladder and urethra (extramural) until they empty into the distal urogenital system.71 The anatomy of the distal segment of the ectopic ureter can be complex with multiple openings and ureteral troughs having been reported.145 Ureteral ectopia is often associated with other urogenital abnormalities, including hydroureter; small, misshapen, or absent kidneys; and vestibulovaginal abnormalities such as paramesonephric remnants.26,74,102,108 Continuous or intermittent incontinence is usually first reported when the animal is young and fails to house-train but occasionally does not begin until later in life.71,74 Even when ectopic ureters are bilateral, most animals produce a urine stream during conscious micturition, possibly because of retrograde urine flow into the bladder in some cases.71,73 Incontinence may be positional, worsening when the animal is recumbent.108 Dogs are more commonly reported than cats (see below), and female dogs are up to 20 times more commonly diagnosed with this abnormality than males (specifics regarding male ureteral ectopia are presented below).69,71 Ureteral ectopia as a cause of urinary incontinence was diagnosed in 217 dogs over a 20-year period at one institution in Great Britain.75 Breeds that were at significantly increased risk included Skye terriers and golden and Labrador retrievers.75 A survey of United States veterinary schools (228 affected dogs) listed Siberian huskies, Newfoundlands, bulldogs, West Highland white terriers, fox terriers, and miniature and toy poodles at increased risk.69
Ureters
Anatomy
Topographic Anatomy
Blood Supply and Innervation
Microscopic Anatomy
Ureteral Obstruction
Feline Ureterolithiasis
Medical Treatment
Lithotripsy
Presurgical Considerations
Localizing the Ureterolith
Surgery
Complications
Canine Ureterolithiasis
Treatment
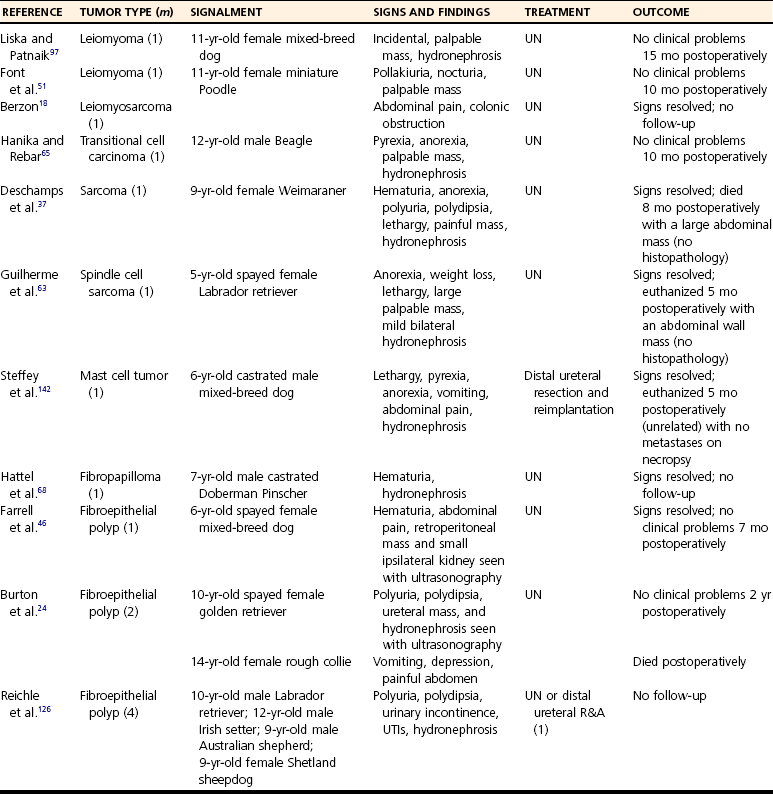
Ureteral Neoplasia
Ureteral Trauma
Ureteral Injury Associated With Ovariohysterectomy
Injury Secondary to Blunt Trauma
Ureteral Ectopia
Clinical Signs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ureters
Only gold members can continue reading. Log In or Register to continue