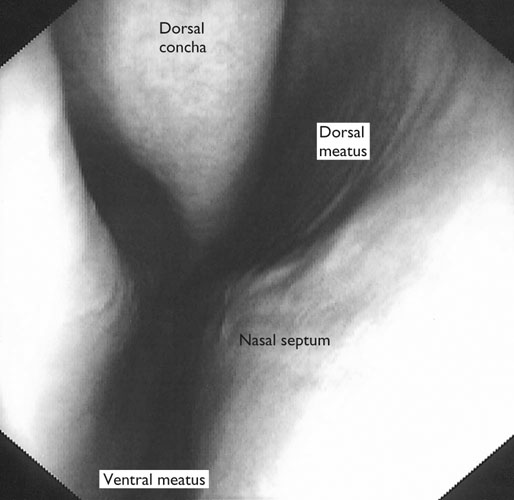
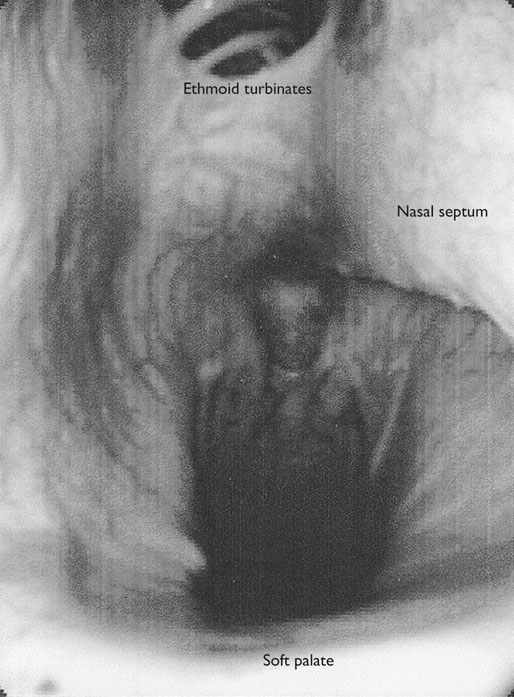
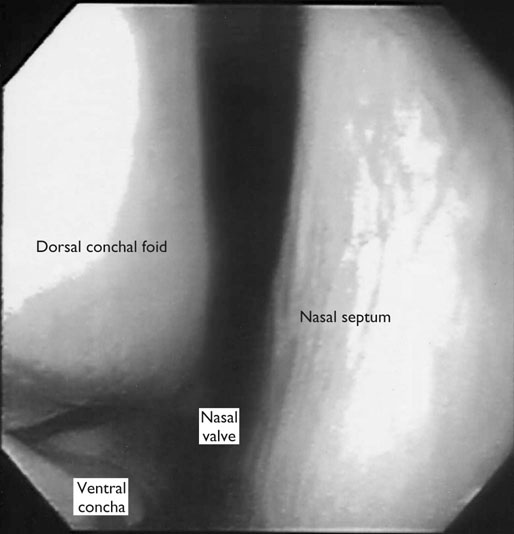
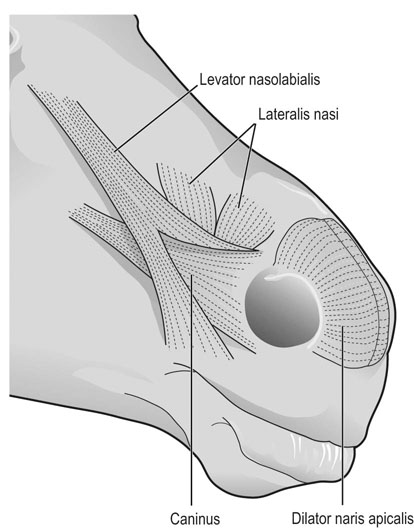
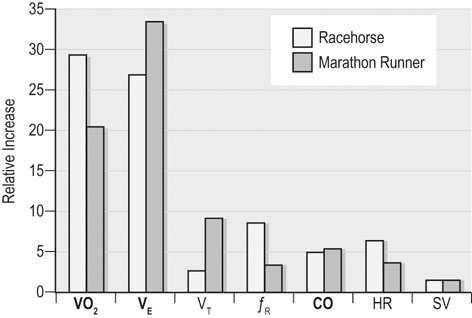
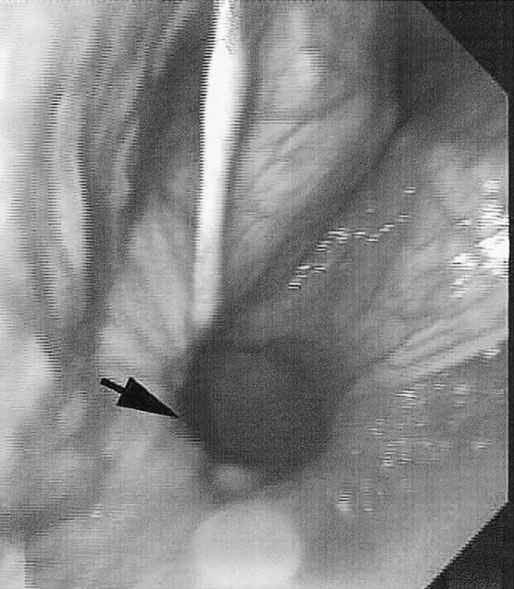
At maximal exertion, Thoroughbred and Standardbred racehorses have a maximal oxygen uptake (VO2max) of approximately 160 mL/kg/min which is 40 times the value at rest.1,2 This increase is far higher than the six to eight fold increase found in endurance trained human athletes and the tenfold increase seen in other mammals.2,3 The delivery of oxygen for this uniquely high rate of consumption requires a number of specific cardiovascular and respiratory responses. Resting oxygen consumption (VO2) in the horse (4.7 mL/kg/min2,4 is similar to that in other mammals.5 As the level of exercise increases so does VO2, and this relationship is linear until maximal oxygen consumption is reached (VO2max) when further increases in speed do not elicit further increases in VO2 and the extra energy required must be provided by anaerobic pathways.5,6 In horses, training can produce a 23% increase in VO2max.7 To maximize locomotor efficiency, each equine gait has an optimal speed at which oxygen consumption is lowest per distance unit traveled.8 In order to accommodate the tremendous oxygen demand of skeletal muscles during such intense exercise, the horse increases its minute ventilation by up to 40 fold. To provide this increase in minute ventilation, tidal volume and respiratory frequency both increase significantly4,7 (Fig. 25.1). High airflow rates required to meet this ventilatory demand are created principally by diaphragmatic contraction, which produces driving high pressures within the upper airway. As the horse is an obligate nasal breather and rarely breathes orally, the horse’s upper airway must quickly prepare for these large changes in airflow and pressures by dilating and becoming more rigid or less compliant. Such accommodation is achieved by the basic structure of the upper airway and synchronous and coordinated contraction of upper airway muscles and constriction of capacitance vessels within the mucosa of the upper airway.9,10 Obligate nasal breathing is a term used to describe a physiological obligation or predisposition to breathe through the nose as opposed to the mouth. It has been suggested that obligate nasal breathing is an adaptation especially useful in prey species, as it allows an animal to feed while preserving their ability to detect predators by scent.11 To accomplish this, the soft palate is tightly apposed to the base of the larynx, such that there is no communication between the oropharynx and the nasopharynx. Oral breathing does occur in horses, but only in the presence of anatomical abnormalities or disease conditions such as dorsal displacement of the soft palate.12–14 The horse cannot breathe nor exercise normally in this state. The resting horse is unusual as it has biphasic exhalation and occasionally a biphasic inspiration.15–18 This flow pattern is lost at exercise or with excitement.18 At rest, the horse breathes around its functional residual capacity (FRC) so that inhalation results in a lung volume higher than FRC and exhalation a lung volume lower than FRC. Equine FRC is approximately 25 L.19 At walk and trot, respiratory frequency is not related to stride frequency.15,20 At the canter and gallop, stride frequency and respiratory frequency are synchronized in a 1 : 1 ratio in fit horses 15,21,22 with the stride lagging approximately 80° behind the respiratory cycle at the canter and 54° at the gallop.15 The synchronization of gait and respiration, with inhalation when the forelimbs are non-weight bearing and exhalation during the support phase, can offer some mechanical advantage.21 Despite this, respiratory muscles work harder during exercise.23,24 In addition, although thoracic expansion occurs at rest, walk, trot and slow canter, at faster speeds there are minimal changes of thoracic circumference with ventilation.25 During swallowing or sighing, normal horses may alter this 1 : 1 ratio.21 In addition, horses may occasionally take a ‘big respiratory cycle’ (BRC) with a duration of approximately two strides.26,27 The incidence of BRCs decreases with training and they can produce improvement in pulmonary gas exchange.27 Respiratory minute volume (VE) in horses is approximately 67 L/min at rest, rising 27 fold to 1800 L/min at exercise.4 At low speeds (up to 6 m/s), this is mainly due to an increase in respiratory frequency (Rf) which rises from 14 breaths/min to 120 breaths/min.4,28,29 Above this speed, there is little increase in Rf, as stride and respiratory cycle are linked, and further increases in VE are due to an increase in tidal volume. Tidal volume (VT) is approximately 5 L at rest and this increases to 14–18 L at maximal exercise.4,29,30 Expiration (E) is shorter than inspiration (I) with an E : I ratio of between 0.96 and 0.99.29,30 A number of clinical studies have evaluated the airway flows and pressures generated by horses at maximal exercise. Peak inspiratory flow at rest is approximately 3.5 L/s 4 and this rises dramatically at exercise to 65–75 L/s.4,29–31 Similarly, peak expiratory flow at rest is 5.7 L/s rising to 60–80 L/s at exercise.4,28–30 These high flow rates are associated with high tracheal pressures both during inspiration and expiration. Peak inspiratory tracheal pressure has been reported as -18 mmHg to -37 mmHg for clinically normal horses at maximal exercise.28,32,33 A computational flow model has recently been used to demonstrate that the greatest drops in airway pressure occur at the nostrils and the rostral aspect of the nasopharynx during inhalation.34,35 This area is susceptible to collapse during inhalation, as it receives only muscular and not bony support. This leads to a reduction in cross-sectional area at this level during inhalation and so the pressure drop. Peak expiratory tracheal pressure has been reported as 6 mmHg to 20 mmHg for clinically normal horses at maximal exercise.28,29,33 During expiration, airway pressure is greatest over the dorsal surface of the soft palate.34,35 High tracheal flow rates and the complex geometry of the upper respiratory tract mean that flow is always in the turbulent range. Tracheal flow in the horse is always turbulent with a Reynolds number (Re) at rest of about 8000.36 Reynolds number (Re) is a dimensionless number that is the ratio of the inertial force to the viscous force in a fluid:37 where ρ is the density, v the velocity, and µ is the viscosity of the fluid. L is the diameter of the tube. Turbulent flow occurs at high Reynolds numbers and is characterized by irregular flow with random eddies and vortices. Airflow in tubes such as the trachea becomes turbulent as airflow velocity increases.38 Flows with Reynolds numbers above 2300 are generally considered turbulent.37 At maximal exercise, the Reynolds number of inspiratory flow can reach 78 000 in vivo.36 A computational flow model has suggested Re as high as 131 000 34 in the horse at exercising airflows. Under these turbulent conditions, pressure changes in approximate inverse proportion to the fourth power of the radius (Poiseuille’s Law). Clinically this is important as small decreases in airway cross-section (for example due to vocal fold collapse) produce profound increases in negative inspiratory pressure which can lead to a cycle of partial collapse, more negative pressure and consequent further collapse. Resistance to airflow produced by the upper airway during inspiration is approximately twice that during expiration and resistance produced by the nose is approximately twice that at the larynx.24,39 The nasal passages (26–76%), followed by the larynx (12–30%), produce the greatest resistance to flow within the upper airway.35 The peak resistance to airflow is termed impedance. Inspiratory upper airway impedance (ZI) at exercise in experimental horses is 0.38 to 0.46 mmHg/L/s.29,30,40 Expiratory impedance is 0.16 to 0.38 mmHg/L/s.29,40 Adaptive responses to an increase in airway impedance include decreased respiratory frequency, increased driving pressure and increased inspiratory time.30,33,40 It is important to note that studies measuring airflow and impedance require the use of a nasal mask and this has been shown to affect airway mechanics.41 The position of the horse’s head and neck does affect upper airway flow mechanics in exercising horses.42 Head and neck flexion may cause the airway to be more compliant, because tissues, such as the pharyngeal walls and soft palate, bulge into the airway, or because the nasopharynx shortens with this maneuver. Horses with most types of obstructive upper airway dysfunction show more severe signs of respiratory distress and exercise intolerance while exercising with the head and neck flexed rather than in a neutral or extended position. During intense exercise, multiple stimuli trigger contraction of upper airway dilating muscles, including chemical stimuli such as hypercapnia and hypoxia, limb movement, central locomotor-linked cortical influences, receptors located in the lower airways, and upper airway sensory receptors.43–50 The laryngeal mucosa has an abundant supply of sensory receptors that control a complex pattern of respiratory reflexes that influence the patency of the upper airway and the pattern of breathing.51 These receptors are mechanoreceptors, chemoreceptors and cold receptors that line the mucous membranes and deeper tissues of the nose, nasopharynx, and larynx.52–54 The mechanoreceptors can be further divided into pressure (64%), ‘drive’ (21%) and flow (15%) receptors.51 The ‘drive’ receptors are stimulated by the activity of upper airway muscles.51 These receptors receive afferent innervation from branches of the trigeminal, glossopharyngeal, and vagus nerves.55,56 Receptors in the nasopharynx are innervated by branches of the glossopharyngeal and trigeminal nerves.51 These receptors are principally tactile receptors and stimulate the gag response, important in airway protection. Especially relevant to dilation and stability of the upper airway during exercise are the pressure receptors. Pressure receptors account for 60% of the sensory receptors within the laryngeal mucosa in horses, which is similar to other species.51,57 These receptors are innervated by the internal branch of the cranial laryngeal nerve which runs through the thyroid notch at the dorsorostral aspect of the thyroid cartilage.51,52,55,58,59 Through this afferent loop, negative pressure within the upper airway increases the activity of the genioglossus, posterior (dorsal) cricoarytenoid, cricothyroid, nasolabial, sternothyroid and sternohyoid muscles.59,60 They are stimulated during upper airway obstruction, when large collapsing pressures are produced in the upper airway, and they provide afferent information to the central nervous system, signaling contraction of upper airway muscles to resist dynamic collapse in the upper airway. In horses, negative pressure stimulates increased electromyographic activity in the cricoaryteneoideus dorsalis muscle, the primary laryngeal abductor.57 During incremental exercise testing the palatinus, palatopharygeus, hyoepiglotticus, sternohyoideus and sternothyroideus, geniohyoideus, and genioglossus muscles all had increasing levels of electromyographic activity as treadmill speed increased, and upper airway pressures became more negative.61–63 Topical anesthesia of the luminal surface of the larynx or bilateral superior laryngeal nerve sectioning markedly reduces the response to changes in upper airway pressure and upper airway muscle activity in laboratory species and people.49,56 In horses, topical anesthesia of the laryngeal mucosa results in increased inspiratory upper airway and nasopharyngeal impedances.64 The dynamic upper airway obstruction was caused by nasopharyngeal collapse due to decreased skeletal muscle support. Following topical anesthesia of the laryngeal mucosa, horses exhibit dorsal displacement of the soft palate and nasopharyngeal collapse during endoscopic examination at rest (Fig. 25.2) and varying degrees of nasopharyngeal collapse during incremental treadmill exercise.64 These results suggest that local sensory receptors in the upper airway of horses, as has been shown in laboratory species, contribute to upper airway patency and that disrupting the sensory component of the local reflex that controls contraction of upper airway muscles can cause dynamic upper respiratory obstruction in horses. Because negative pressure induces contraction of upper airway muscles, a nasal occlusion test was developed in horses to challenge the upper airway with more negative pressures during resting videoendoscopic examination, in order to mimic pressure changes that might occur during intense exercise. Peak tracheal inspiratory pressure during nasal occlusion (−24.9 ± 3 cm H2O) is not significantly different from peak inspiratory pressure while horses exercise at HRmax (−25.6 ± 2.7 cm of H2O), and peak pharyngeal inspiratory pressure is significantly more negative (−28.9 ± 4.9 cm of H2O) during nasal occlusion than while horses exercise at HRmax (−17.5 ± 2.1 cm of H2O).65 These data indicate that nasal occlusion in standing horses results in pharyngeal and tracheal inspiratory pressures that equal or exceed those that are generated during exercise at HRmax, making it a potentially useful test for evaluating the activity of laryngeal and pharyngeal muscle function. However, anecdotally, horses that exhibit varying degrees of nasopharyngeal collapse at rest frequently have normal airway function during treadmill exercise. As well, horses that readily displace their soft palates at rest frequently displace during treadmill exercise, but the correlation between displacement at rest and during treadmill exercise is not a strong one. Other sensory receptors within the upper airway include flow and drive receptors. Drive receptors represent 20% of laryngeal sensory receptors in horses.51,57 These receptors respond to changes in airway deformation, such as collapse, muscle contraction and movement of the laryngeal cartilages.51 Flow receptors are temperature-sensing receptors.51 These receptors sense cool air temperatures, which occur as air flow increases. The majority of these receptors are responsive during inspiration, but some (approximately 20%) respond during exhalation, and a small population of the airway sensory receptors is active both during inspiration and expiration.51 The horse’s nose includes the paired external nares, the nasal cavities, and the paranasal sinuses. The nostril has two compartments: a dorsal blind sac called the nasal diverticulum and the ventral part, which is the true nostril.66 The alar fold divides the nostril into the dorsal and ventral parts. The nasal cavity is divided in half by the nasal septum and vomer bone. Each nasal cavity has a dorsal and ventral nasal concha, which divide the cavity into dorsal, ventral, middle, and common meatus (Fig. 25.3).66 The ethmoid turbinates project from the ethmoid bone in the caudal part of the nasal cavity (Fig. 25.4). The nasal valve is the narrowest point in the nasal cavity and, thus, is a major contributor to nasal resistance.9 This region is caudal to each nostril and immediately rostral to the nasoincisive notch within the dorsal meatus. It is bound medially by the nasal septum, ventrally by the concha, and dorsolaterally by the skin and dorsal conchal fold (Fig. 25.5).66 Expansion of the nasal valve during exercise occurs by constriction of capacitance vessels and contraction of muscles that pull the skin taught at the dorsal aspect of the notch. The respiratory portion of the nasal cavity has a vascular submucosa, which contains a rich vascular plexus. This plexus is concentrated on the ventral portion of the nasal septum and ventral meatus and is important for warming and humidifying air. The capacitance blood vessels in the airways, especially those lining the nasal mucosa, are innervated by sympathetic, parasympathetic, and peptidergic systems that regulate blood flow.10 This extensive vasculature is responsible for warming and humidifying inspired air. When these vessels are dilated, the sinuses and nasal passages become engorged with blood, resulting in airway narrowing and increased airway resistance. Such nasal congestion is a cause of airway obstruction and poor performance in horses with Horner’s syndrome. During exercise, sympathetic stimulation causes vasoconstriction, increasing airway dimensions and decreasing resistance to airflow.10 The nasal vestibule is lined by fine hairy skin and there is an abrupt change to non-ciliated nasal mucosa over the base of the ventral nasal concha.18 In obligate nasal breathers such as the horse, increasing nasal patency during exercise is critical to minimizing work of breathing. At rest, the horse’s nostril is shaped like a comma, but as the horse increases its respiratory effort the nostril dilates and becomes circular in shape. Horses can actually decrease their nasal resistance during exercise by increasing nasal volume and flaring their nostrils. Nostril dilation is accomplished by the contraction of four different muscles (Fig. 25.6).66 Contraction of the lateralis nasi dilates the nostril, rotates the conchal cartilages laterally, and expands the nasal vestibule, which forms the floor of the nasal valve.66 Other muscles involved in dilatation of the nostrils include the caninus, dilator naris apicales, and levator nasolabialis. These muscles are all innervated by the facial nerve (VII). Contraction of the caninus or dilator naris lateralis muscle helps expand the lateral aspect of the nostrils.66 Dilator naris apicales is an unpaired muscle that lies between the nostrils and aids in dilatation of the nostrils.66 Levator nasolabialis dilates the nostrils and also elevates the maxillary lip and commissures.66 Horses with dysfunction, weakness, or lack of contraction of one or more of these muscles will likely have dynamic nasal obstruction that may limit performance. The nasopharynx is a musculomembranous unit that functions during breathing, deglutition, and vocalization and connects the nasal cavity to the larynx. It is attached to the pterygoid, palatine, and hyoid bone, and to the arytenoid, cricoid, and thyroid cartilages by nasopharyngeal muscles that cause pharyngeal dilation and constriction.67 The nasopharynx is not directly supported by cartilage or bone, yet contraction of these pharyngeal muscles allows the nasopharynx to withstand large changes in intraluminal pressures that occur during tidal breathing at rest and during exercise. Such activation of these muscle groups is synchronous with breathing and this synchronization is coordinated by multiple stimuli.46,68 These same muscles are also important during deglutition. This dichotomy of action, contracting the pharyngeal walls during swallowing and dilating the airway during breathing, seems contradictory. But these muscles are uniquely situated to perform both activities, because the pharynx is a conduit for both food and air. The nasopharynx is lined by pseudostratified columnar ciliated epithelium.18,69 Elastin fibers are present in the nasopharyngeal submucosa and are more prevalent caudally.69 The equine nasopharynx at the level of the soft palate has a cross-sectional area of approximately 2430 mm2.34 Exhalation results in significant dilation of the nasopharynx to 3920 mm2 at the same level.70,71 The soft palate completely divides the pharynx into nasal and oral compartments in the horse. Because the horse is an obligate nasal breather, it is critically important that the soft palate remains ventral to the epiglottis, except during swallowing, to allow unimpeded nasal breathing. The soft palate extends caudally from the hard palate to the base of the larynx and consists of the oral mucous membrane, which contains ductile openings of the palatine glands, the palatine glands, the palatine aponeurosis, palatinus and palatopharyngeus muscles, and the nasopharyngeal mucous membrane.67 The caudal free margin of the soft palate continues dorsally, on either side of the larynx, forming the lateral pillars of the soft palate. These pillars unite dorsally, forming the posterior pillar of the soft palate or the palatopharyngeal arch. The position of the soft palate is determined by the coordinated activity of groups of antagonistic muscles which include the levator veli palatini, tensor veli palatini, palatinus, and palatopharyngeus muscles (Fig. 25.7).72,73 The levator veli palatini muscle attaches to the petrous part of the temporal bone and the lateral lamina of the guttural pouch. It travels along the lateral wall of the nasopharynx and terminates within the soft palate. A branch of the pharyngeal branch of the vagus nerve innervates this muscle.67 It acts to elevate the soft palate during swallowing and vocalization. The action of the levator veli palatini muscle can be seen during endoscopic examination of the upper airway when the gag reflex is stimulated (Fig. 25.8). A ‘sling’ forms within the nasopharynx as the nasopharynx contracts into a sphincter. The tensor veli palatini is a flat, fusiform muscle that, like the levator, attaches to the petrous part of the temporal bone, the pterygoid bones, and the lateral lamina of the guttural pouch.67 Its tendon is reflected around the hamulus of the pterygoid bone, where it is lubricated by a bursa. The tendon then ramifies in the palatine aponeurosis.67 It receives motor innervation from the mandibular branch of the trigeminal nerve. Contraction of this muscle tenses the palatine aponeurosis and, therefore, the rostral portion of the soft palate, and depresses this portion of the soft palate toward the tongue.67
Upper airway function of normal horses during exercise
Introduction
Obligate nasal breathing
Basic upper airway mechanics
Ventilation
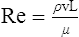
Head position
Neuromuscular control of upper airway function

Nasal occlusion
Muscular anatomy and function of the upper airway
The nose
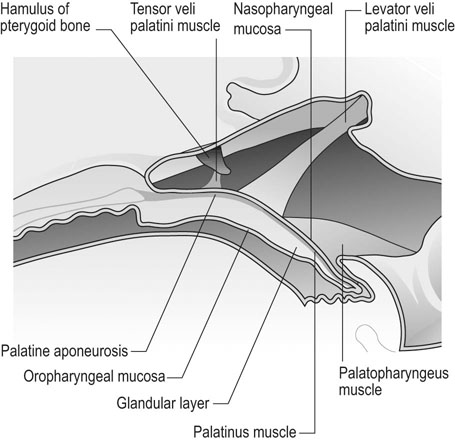
The nasopharynx
Soft palate
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Upper airway function of normal horses during exercise