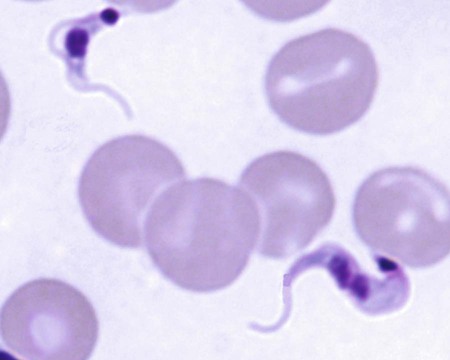
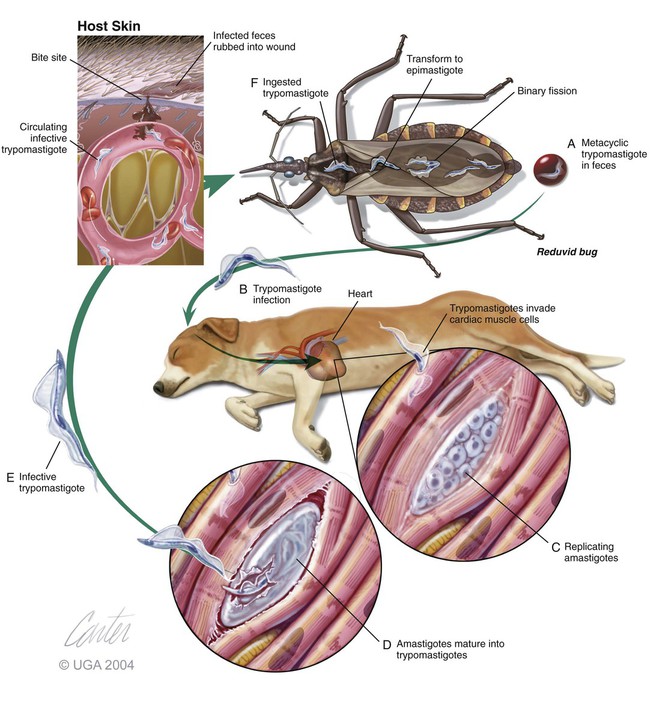
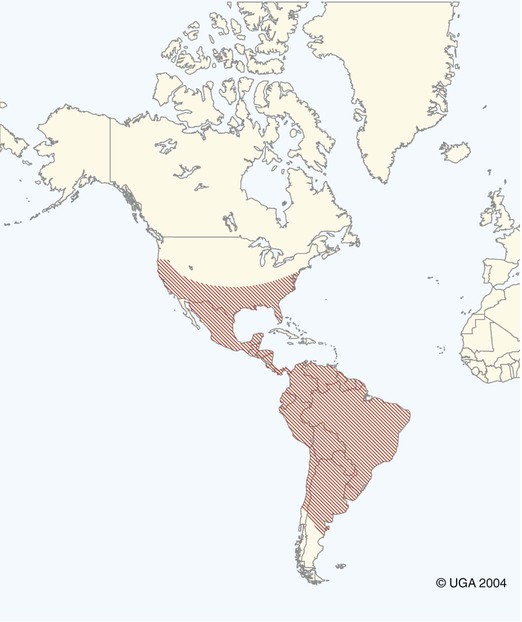
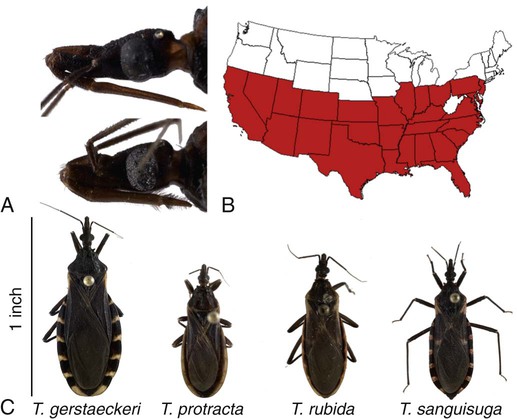
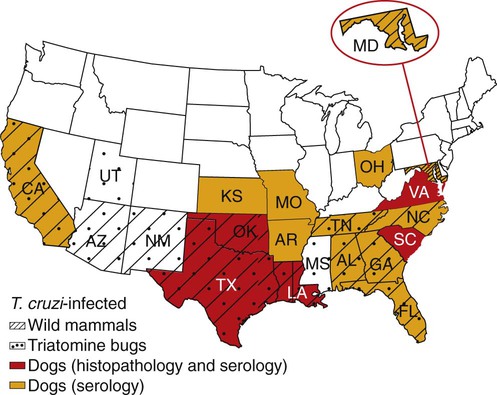
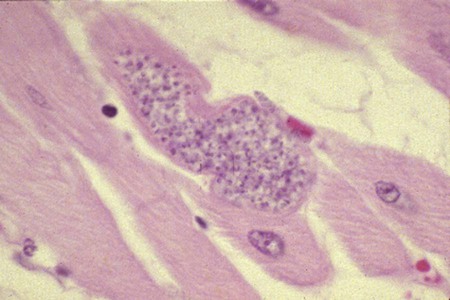
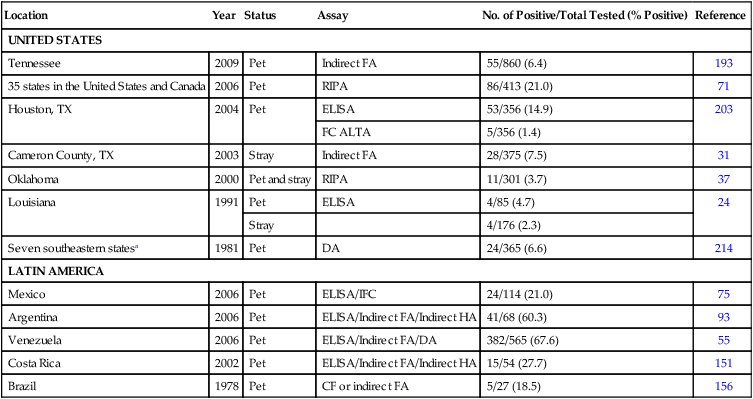
Karen F. Snowden and Sonia A. Kjos Trypanosoma cruzi, the etiologic agent of American trypanosomiasis or Chagas’ disease, exists in several morphologic forms.217 In the mammalian host, the extracellular spindle-shaped trypomastigote or blood form is 15 to 20 µm long, with a central vesicular nucleus. A single, free flagellum originates from a basal body near the large posterior subterminal kinetoplast and an undulating membrane passes along the body to project anteriorly (Fig. 72-1). The second mammalian form is an oval intracellular amastigote approximately 1.5 to 4.0 µm in diameter that contains a large, round nucleus and rodlike kinetoplast similar to that of Leishmania (see Fig. 73-3). Epimastigotes, the third morphologic form, are found in the triatomine bug vector (Hemiptera: Reduviidae), commonly known as the kissing bug. This flagellated and somewhat pleomorphic form (15 to 20 µm) has a kinetoplast situated adjacent to the nucleus with a short undulating membrane and a single anterior flagellum. Stercorarian transmission occurs when the infected bug defecates on or near the host during or shortly after feeding and the fecal material containing trypomastigotes is subsequently rubbed into the bite wound, skin abrasions, or mucosal tissue (Fig. 72-2). Oral ingestion of infected insects will cause infection in opossums, raccoons, and wood rats and is a probable route of infection in dogs.151,188,188 Other less common sources of infection in humans and possibly in dogs include blood transfusions, congenital factors, or ingestion of meat or ingestion of milk from infected lactating animals.29 Trypomastigotes usually enter macrophages and myocytes, either locally or systemically, after hematogenous spread. Once they are intracellular, trypomastigotes transform into amastigotes, which multiply by binary fission. These amastigotes transform into trypomastigotes before rupture of and release from the cell. Rapid intracellular multiplication cycles ensure a rapid rise in parasitemia before effective immunity develops. The bug vector becomes infected by ingesting circulating trypomastigotes, which transform to epimastigotes and multiply by binary fission. Transformation of the epimastigotes back into trypomastigotes occurs in the vector’s hindgut before the trypomastigotes are passed in the feces (see Fig. 72-2). T. cruzi infects humans and a wide range of domestic and wild mammalian species in the Western Hemisphere (Fig. 72-3). With an estimated 8 million human infections and 110 million at risk, it is considered the most important parasitic disease in the Americas.169 The greatest burden of Chagas’ disease was initially recognized in South American countries, particularly Argentina, Bolivia, Brazil, and Columbia. Infection in humans and domestic and wild mammals has also been documented in Central American countries, including Mexico, where human prevalence is estimated to be 1.6 million cases, and seroprevalence in dogs is reported as high as 21% in certain areas.13a,75,96 Infection has also been documented on Caribbean Islands.104,191 Although transmission of the parasite can occur by blood transfusion, organ donation, ingestion of contaminated food or drinks, or transplacentally, greater than 80% of all human cases are caused by vector-borne transmission.68 Members of the Triatominae subfamily, which are obligate blood feeders in all post-egg stages, are the biological arthropod vectors of T. cruzi. Triatomine bugs can be distinguished from other reduviids by the location of antennae situated in front of the eyes, and piercing/sucking mouthparts (rostrum), with or without bristles, that gradually taper to the apex (Fig. 72-4, A). The rostrum lies flat at the attachment point to the head rather than curved as in predaceous reduviids. Triatomine bugs feed on a broad range of vertebrate hosts including mammals, birds, and reptiles, but only mammals are susceptible to infection with T. cruzi. Although all triatomine species are considered potential disease vectors, only species that have adapted to living in or near domestic structures are of primary public health importance. The usual transmission of T. cruzi in endemic countries depends on the confluence of reservoirs, vectors, parasites, and hosts (humans or animals) in a single habitat. Two extremes of vector behavior have been noted: those that are habitually domiciliated and those that are habitually sylvan; many species are intermediate in behavior. Because of this variation, vectors tend to have either domestic or sylvatic cycles, with occasional crossover between the two. Molecular and morphologic data suggest that the establishment of the T. cruzi disease cycle in the United States, including the parasite, bug vectors, and susceptible mammalian hosts, predates the arrival of humans to this region.38,201 The bugs are inhabitants of most southern states from coast to coast (Fig. 72-4, B) and in Hawaii. Eleven species of triatomine bugs have been documented in the United States, 10 of which belong to the Triatoma genus (gerstaeckeri, incrassata, indictiva, lecticularia, neotoma, protracta, recurva, rubida, rubrofasciata, and sanguisuga) (Fig. 72-4, C). The remaining species is Paratriatoma hirsuta. All Triatoma species, with the exception of incrassata, have been found naturally infected with T. cruzi, and P. hirsuta has shown to be a competent vector in experimental studies.127 T. cruzi–infected bugs have been reported from the majority of southern states (Fig. 72-5). The data on prevalence of T. cruzi in U.S. bug vectors are primarily derived from specimens collected in Texas, California, and Arizona, with rates ranging from 17% to 51%, 14% to 40%, and 7% to 41.5%, respectively.* Encounters between humans and triatomine bugs inside houses in the United States, including bite events, were recorded as early as the late 1800s.116 The species collected most frequently from houses include Triatoma gerstaeckeri (Texas, New Mexico), Triatoma protracta (central to western-southern states), Triatoma rubida (central to western-southern states), and Triatoma sanguisuga (central to eastern-southern states) (see Fig. 72-4, C).121,168,232,236 In addition to their role as disease vectors in the United States, triatomine bugs are capable of inducing a severe allergic reaction in some individuals.78,131,131 The prevalence of human Chagas’ disease in the United States due to vector exposure is low, with only 7 reported cases (4 in Texas, and 1 each in California, Tennessee, and Louisiana).† The largest contributing factors to low human prevalence are very low levels of triatomine colonization in houses and delayed defecation patterns by native triatomine species.123,177 Most triatomine bugs found inside houses in the United States are adults. Immature stages recovered from inside houses, which would suggest colonization in or near the house, are reported infrequently. In contrast to important South American vector species, some United States species have been reported to defecate less frequently on the host in experimental studies, suggesting that they are less efficient vectors of T. cruzi. This reduced contact between humans, triatomine bugs, and infectious bug feces results in lower risk of human disease transmission. Because of Chagas’ disease blood transfusion and organ transplantation cases in the United States,50 the Food and Drug Administration approved a diagnostic blood screening test in 2007 for voluntary use by United States blood banks.77 As of 2009, confirmed, T. cruzi–positive donors had been identified in 42 states.1 The number of infected immigrants living in the United States is estimated to be greater than 300,000,33 and these individuals account for the vast majority of the infected blood donors. The enhanced surveillance among blood donors has also identified additional United States autochthonous human cases, presumably due to vector exposure.44 Acute and chronic cases of Chagas’ disease have been reported in domestic dogs in the United States from at least 16 states (see Fig. 72-5). The first infections were recognized among 9 dogs of 7 different breeds in Texas in 1972.229 These dogs were distributed across eight different counties in the state, all but one animal were less than 1 year old, and all suffered an acute, fatal illness. Since the initial discovery of canine Chagas’ disease in the United States, several seroprevalence studies among domestic dogs, detecting T. cruzi–specific antibody, have been reported (Web Table 72-1). A sample of Latin American canine seroprevalence data is also provided in the table for comparison. Differences in the methods of antibody detection used and differences in study population size and distribution make the seroprevalence rates, which range from 1.4% to 21% in the United States, difficult to compare. The highest infection rate (21%) was derived from a study of hunting foxhounds residing in 13 states,71 whereas a survey among mixed-breed hunting dogs in Louisiana resulted in a much lower rate (4.7%).24 Among owned dogs in Houston, Texas, infection rates differed considerably for the same study population, depending on the serologic assay used, varying between 1.4% (flow cytometry using trypomastigote-derived antigen) and 14.9% enzyme-linked immunosorbent assay (ELISA, using epimastigote-derived antigen).203 Health status of the study populations varied as well, ranging from hospitalized dogs,214 to clinically healthy dogs,203 to unknown health status.24,31,37,71,193 Age of dogs with positive antibody test results was reported in two of the U.S. studies. A survey of dogs visiting veterinary clinics in Tennessee found that those with positive antibody test results were more commonly between 6 and 10 years of age.193 Similar Latin American canine seroprevalence studies show the same trend of increasing seropositive results with increasing age.75,93 The higher prevalence among older dogs is likely due to their greater accumulated time for exposure to infected triatomine vectors or other routes of exposure. However, in contrast, hospitalized dogs with seropositive results in one study in the United States were significantly younger than dogs with seronegative results, and 2 of the 7 dogs with positive results were less than 1 year of age.214 In a medical records review of 537 canine Chagas’ disease cases in Texas, 50% of the dogs diagnosed by serologic or histopathologic methods and 50% of the acutely dying dogs were less than 1 year of age.120 As reflected in this study, it is likely that dogs that become infected at a very young age (due to transplacental or transmammary exposure in addition to infected triatomine and mammalian tissue exposures) more often suffer an acute, fatal illness than dogs that become infected later in life. Similar to infection in humans, dogs that survive infection as a puppy or acquire infection at an older age will generally experience a more insidious chronic course of disease that may result in development of significant cardiac manifestations. WEB TABLE 72-1 Seroprevalence Surveys of Trypanosoma cruzi Infection among Domestic Dogs CF, Complement fixation; DA, direct agglutination; ELISA, enzyme-linked immunosorbent assay; FA, fluorescent antibody; FC ALTA, flow cytometry anti–live trypomastigote antibody; HA, hemagglutination; IFC, immunofluorescence flow cytometry; RIPA, radioimmunoprecipitation assay. aFlorida, Georgia, Louisiana, North Carolina, South Carolina, Virginia, West Virginia. T. cruzi infection has been documented in a wide variety of dogs including 48 breeds in the Texas medical records study120 and 41 breeds in the Tennessee seroprevalence study.193 Presumably, the higher prevalence in sporting and working groups is due to lifestyle factors that increase the dogs’ exposure to infected triatomine vectors or mammalian tissues, such as confinement in outdoor kennels or through hunting activities, rather than due to breed predilection for the pathogen. Although cross-reactivity with Leishmania spp. is a concern in T. cruzi serologic surveys, histopathologic or parasitologic confirmation of T. cruzi infection has been reported in dogs from at least five states, providing further evidence of active and widespread canine Chagas’ disease transmission in the United States (see Fig. 72-5).29,37,120,162,205 As in humans, transplacental transmission in dogs may significantly contribute to the prevalence of Chagas’ disease in this species. Transplacental and transmammary transmission was documented in experimentally infected dogs in very early studies.144,206 In experimental studies in rodents inoculated with the dog strain of T. cruzi, transplacental transmission was reported in acutely parasitemic animals.152 Natural infection in eight pups born to a Walker hound female dog in Virginia with a positive serum antibody titer was suggestive of transplacental or transmammary infection due to the lack of vector presence or exposure to infected wild mammalian tissues.29 Data from canine field studies in Argentina showed that 10% of offspring born to dams with positive serum antibody titers were also infected as determined by serologic testing.47 T. cruzi infection in wild mammals has been reported from most areas of the southern United States (see Fig. 72-5) and other regions of the Americas where the disease is endemic. The principal sylvatic reservoir hosts of T. cruzi in the southern United States are opossums, raccoons, and armadillos.* Similarly, in Maryland,225 Oklahoma,109 North Carolina,113 South Carolina,238 and Georgia,175,178,178 raccoons and opossums are the main reservoir hosts. In the southwestern states, T. cruzi infection has been detected in various rat, mouse, and squirrel species.159,233,233 Triatomine bug species distributed from the western half of Texas to California are frequently found in the nests of wood rats. Consequently, a significant percentage of wood rats in field surveys have been found to be infected with T. cruzi and probably serve as reservoirs in those regions.41,177,177 Other wild mammal species have been reported to be infected with T. cruzi, including coyotes, gray foxes, skunks, and badgers. Because experimental inoculation of T. cruzi isolates from opossums and armadillos into dogs produces a disease similar to that described in naturally acquired cases of acute and chronic canine trypanosomiasis, dogs in natural settings are likely infected with the same parasite strains as those sylvatic hosts.24–26 Future genetic comparisons of isolates will help confirm this assumption. Inoculation of T. cruzi into mice using strains from wildlife and dogs in the United States induces mild illness compared to similar inoculation of similarly collected strains from South America188a indicating the former strains are of lower virulence. Another closely related organism, Trypanosoma caninum has been isolated from the skin of dogs.67a Some animals had increased anti-Leishmania sp. antibodies which can indicate a possible cross-reactivity for serodiagnosis. Further studies are needed to determine the clinical significance of infection with T. caninum. Trypanosoma evansi is another member of the Trypanosoma genus with a wide geographic distribution including Africa, Australasia, and limited regions in Brazil, South America.9,179 The parasite infects a wide range of mammalian hosts including domestic and wild hoofstock such as cattle, buffalo, and horses; a number of small wild mammals such as rodents, coatis (Nasua nasua), capybaras (Hydrochaeris hydrochaeris), and several bat species; and occasionally dogs.64,99,99 Cyclical biologic transmission occurs through the bite of infected tsetse flies (Glossina spp.); this is an important means of transmission in Africa where these flies are endemic (see African Trypanosomiasis, later). In the Americas, other means of transmission are suspected. The parasite is also transmitted mechanically by blood-sucking flies, mainly of the genus Tabanus, and direct transmission by vampire bats has also been described.100 Under experimental conditions, oral transmission of the parasite has been documented in dogs and in mice by feeding heavily parasitized blood and tissues.181 In limited geographic areas in Brazil, endemic regions for T. cruzi, T. evansi, and Leishmania chagasi overlap. Because dogs may serve as hosts for all three of these parasites, distinguishing among these parasitic infections can be problematic (see also Chapter 73, Leishmaniases).218 Additionally, co-infection by L. chagasi and T. evansi have been reported, further complicating the diagnosis of these parasites in this region.199 For a further discussion of T. evansi infection, see African Trypanosomiasis, later in this chapter. A Trypanosoma species different from T. cruzi or other members of the genus was isolated from culture of skin from a domestic dog in Rio de Janeiro, Brazil.137 It was provisionally named T. caninum; however, the pathogenic significance of this finding is uncertain. The host-parasite interactions in T. cruzi infections are complex, and it is likely that both parasite and host response contribute to the progressive cardiac damage.4 In experimental canine infections, the intensity of inflammatory infiltrate and fibrosis in the heart varied based on the strain of the parasite in both acute and chronic phases of infection.25,87,87 In medical literature reporting pathologic changes in acute Chagas’ disease in humans, a microangiopathy has been described, and an ischemic vascular component to the cardiac destruction has been suggested.4 The mechanism by which progressive cardiac damage occurs may be linked in part to host immune responses to the parasite. In experimentally infected beagles, high levels of the anti-inflammatory cytokine interleukin-10 were consistently detected in animals that showed less severe cardiac pathology during chronic infection.87 The role of various anti-parasite immunoglobulin subclasses in contributing to cardiac damage is unclear. In one study, an elevated IgG1 isotype was reported in dogs with an increased cardiac index and myocarditis.56 Conversely, in another study, low IgG1 levels correlated with cardiomegaly in chronically infected dogs.88 In a review of Chagas’-associated human pathology, it was hypothesized that a balance of cell-mediated immune response and inflammatory response were maintained in the indeterminate stage of infection.4 The progressive cardiac damage and inflammation in chronic disease was attributed to mediation by cytotoxic CD-8 lymphocytes, although the mechanism of cell-mediated myocardial damage was not clearly elucidated. An autoimmune component in cardiac damage has been proposed, especially because there is a lack of correlation between intracellular amastigote numbers and the degree of chronic myocarditis with fibrosis. Protective immunity was not documented in experimentally infected dogs that received five repeated infections over a 3-year period. Over time, dogs developed diminishing parasitemias and increased antibody titer results, with a progressive myocarditis similar to control animals.134 The course of T. cruzi infection in dogs appears to follow the cardiac disease pattern in humans, including an acute phase, followed by an “indeterminate” or latent phase with normal clinical presentation, and a chronic phase of cardiac damage.4 Acute disease occurs mainly in young dogs and is sudden in onset, with signs of myocarditis and cardiac arrhythmias. In one study reviewing records of dogs with histopathologically confirmed T. cruzi infections in Texas, 50.5% of the infected dogs were less than 1 year of age.120 At the cellular level, the cause of the myocarditis is due to cell death and the release of inflammatory mediators of several kinds as parasites replicate and lyse the cardiomyocytes.4,18 Signs referable to acute myocarditis, such as sudden collapse and death of a previously normal young dog, have been reported frequently. Pale mucous membranes, slowed capillary refill time, weak pulse with deficits, tachyarrhythmia, and terminal hypothermia and respiratory distress are common. In one study reviewing 86 infected dogs, 42% died acutely, and the diagnosis of Chagas’ disease was determined by postmortem histopathologic studies.120 Many infected dogs that do not die suddenly develop ascites, hepatomegaly, and splenomegaly associated with heart failure. In addition to cardiac manifestations, dogs may exhibit other signs of acute disease. Generalized lymphadenomegaly, anorexia, and diarrhea are common during acute illness and may precede cardiac manifestations. Neurologic signs referable to meningoencephalomyelitis, including paresis, ataxia, and upper motor neuron (hyperreflexive) spinal reflexes, have rarely been described in naturally and experimentally infected dogs.25,32 In adult dogs, these acute manifestations are sometimes less severe, with only transient mental depression and parasitemia.18 The reason for variation in intensity of illness with age is unclear; however, it may relate to inherent age-related host immunity. Survivors of acute myocarditis will become aparasitemic and asymptomatic in what is called the indeterminate or latent phase. During this phase, parasitemias are not detectable. During the long asymptomatic period between acute and chronic disease in the dog, electrocardiographic (ECG) readings may be within reference limits, except for the intermittent occurrence of ventricular arrhythmias, which can be exacerbated by exercise or excitement.15 Sudden death during this stage can occur and is thought to be caused by fatal cardiac arrhythmias. Although not all dogs progress to chronic disease, many develop chronic myocarditis with bilateral cardiac dilatation over a variable length of time. The progress of disease over time has not been well documented in natural infections, but in experimental studies in young dogs, the onset of chronic disease was documented over an 8- to 36-month period postinoculation.25,26 In the chronic state of the infection, cardiac dilatation occurs, ECG abnormalities become more prevalent, and clinical signs referable to right-sided and/or bilateral cardiac failure occur. In experimentally chronically infected dogs, clinical signs included pulse deficits, ascites, pleural effusion, hepatomegaly, and jugular venous congestion.25 In two studies reviewing case records of naturally infected dogs, the most common clinical history and physical examination abnormalities included exercise intolerance, lethargy, anorexia, ascites, respiratory difficulties (tachypnea and coughing), bradycardia, heart murmurs, and hepatomegaly.120,146 Chronic T. cruzi–induced myocardial disease in dogs is sometimes difficult to distinguish from chronic dilative cardiomyopathy of noninfectious origin seen in large breeds of dogs, and those cases may be clinically misdiagnosed until serologic evaluation for exposure or histologic detection of parasites is available.24,28,28 In chronic infections, one sometimes overlooked aspect of disease is the presence of electrical conduction disturbances along with variable cardiomegaly. In a Texas study reviewing case records, 21.3% of dogs had electrical conduction disturbances, and a number of animals were being evaluated for pacemaker implantation.120 A variety of electrical conduction abnormalities have been reported, including atrial fibrillation, ventricular premature contractions, first- and second-degree heart block, and tachyarrhythmias.18,26 Parasite-associated damage to parasympathetic innervation in the heart and subsequent conduction disturbances are probably responsible for the cardiac arrhythmias. In experimental infections in both rats and dogs, a reduction in autonomic neurons has been documented, along with a variable intensity of inflammatory changes in histopathologic studies.89a,132 Trypanosomiasis should be considered in any dog with signs of myocarditis, cardiomyopathy, or electrical conduction disturbance in the heart. Megaesophagus and other “megaviscus” syndromes described in humans with chronic Chagas’ disease have not been described in dogs naturally infected with T. cruzi. Morphometry of the myenteric esophagus was not altered in dogs with experimentally induced chronic T. cruzi infection.133 There is little information available on survival times of dogs. In a review of 11 dogs identified with chronic T. cruzi infection, survival times ranged from 0 to 60 months.146 Two distinct groups were evident: dogs diagnosed at an older age (mean of 9 years) survived between 30 to 60 months, whereas dogs diagnosed at a younger age (mean of 4.5 years) survived a maximum of 5 months postdiagnosis. Field studies in South America have documented significant levels of serum antibody reactivity to T. cruzi in cats and have implicated this species as a potential reservoir in the domestic transmission cycle.93,156 Unfortunately, data on the clinical course of disease in cats are lacking in the published literature. It is possible that Chagas’ disease occurs in cats living in the United States, even though it has not been reported. Serologic testing for T. cruzi antibodies in cats is not generally available in commercial diagnostic labs, complicating detection of infection in this host species. ECG changes during acute disease in experimentally infected dogs are highly variable and include alteration in ST-T segments, T-wave inversion, low-amplitude QRS complexes, positive polyphasic ventricular premature contractions, and first- and second-degree heart block.26 ECG information is valuable in the diagnosis of the dilative cardiomyopathy of chronic disease. Thinning of ventricular walls, large end-diastolic volumes, and low shortening fractions (usually under 20%) have been reported in experimentally infected dogs.26 ECG findings in naturally infected dogs include first-degree heart block, right bundle-branch block, decreased-amplitude QRS complexes, ventricular premature complexes, atrial fibrillation, ventricular tachycardia, and third-degree atrioventricular block.28,146 Thoracic radiography is of value in the diagnosis of pleural effusion, pulmonary edema, and cardiomegaly in both acute and chronic myocarditis.146,195 Echocardiographic abnormalities, reported from eight chronically infected dogs, have included a variable combination of changes such as enlargement of all four heart chambers, regurgitation through the right and left atrioventricular valves, and pericardial effusion.146 In a case report of acute disease in a puppy, echocardiography revealed severe right heart dilatation.195 Hematologic abnormalities are of little specific diagnostic value. Leukocytosis may occur in the acute phase in a small number of cases.195 Alanine aminotransferase activity may be elevated as a result of hepatic hypoxia. In a retrospective study, 5 of 10 chronically infected dogs showed elevated alanine aminotransferase values.146 Elevation in serum troponin I levels have been reported as a non-specific indicator of myocardial damage.18 Creatine kinase (CK) and lactate dehydrogenase activities are rarely elevated during acute disease. Elevations of serum CK (isoenzyme MB) occur but are too variable and transient to be of any diagnostic value in the dog.25 The abdominal effusion is typically a modified transudate, similar to that from cardiac causes.195 Trypomastigotes can be identified in stained blood films of dogs, just before and during acute disease (see Fig. 72-1).195 In experimental infections in puppies, parasites could be detected in circulation as early as 3 days postinoculation, and parasitemia peaked about day 17, approximately at the time that clinical signs of acute myocarditis and lymphadenomegaly appeared.18 In those animals, parasitemia persisted for up to 30 days. However, in chronic infections, parasitemias are often so low that organisms may be difficult to identify during routine examination of Wright-stained blood films. To concentrate parasites and increase the sensitivity of detection, a thick-film buffy coat smear can be stained with Wright’s or Giemsa stain and examined microscopically, or a buffy coat specimen may be examined as a wet preparation for trypomastigote movement.195 Stained lymph-node aspirates or impression smears may contain amastigotes, even when parasitemias are very low.158 Abdominal effusions may contain organisms that can be identified cytologically. Serologic testing for T. cruzi–specific serum antibodies has been used in multiple epidemiologic studies to evaluate the prevalence of infection in dog populations and other mammalian hosts (see Web Table 72-1). A positive result, in association with clinical signs, is considered a standard approach for the diagnosis of Chagas’ disease in dogs. In experimental infections, antibodies are usually first detected by 3 weeks postinoculation, at the time when parasitemias are declining, and positive titers persist for the life of the animal.23 Various antibody detection methods are used by fee-for-service diagnostic laboratories including the indirect immunofluorescent antibody tests and ELISA. Several ELISA tests are commercially available for human use in a number of Latin American countries,167 and some of the tests have been adapted for canine testing. Additionally, radioimmunoprecipitation assays, indirect hemagglutination assays, and flow cytometric assays have been used in research studies with dogs.* These tests are designed to detect and quantitate canine antibodies to T. cruzi; however, there may be confusion due to significant cross-reactivity and cross-infection with the closely related parasite Leishmania.85,215 Depending on the clinical history and geographic location of the animal, positive serum antibody test results for T. cruzi should be confirmed by additional testing for antibodies to Leishmania to determine which organism yields the higher titer.71 Several immunochromatographic point-of-care “dipstick” tests have also been developed for use in humans and in dogs, although their use has been limited. In experimental trials in the United States and in Argentina, these tests performed favorably when compared with other antibody detection methods.45,161,190a These test kits have potential as rapid, simple screening tools in a clinic setting that can be used in conjunction with additional confirmatory tests. Web Appendices 5 and 6 should be consulted for laboratories and test kits, respectively, that are available for performing the serologic testing just described. Polymerase chain reaction (PCR) testing has been used as a tool to confirm the diagnosis of T. cruzi infection in several clinical reports of canine infection.29,158 However, PCR assays for the detection of T. cruzi in blood or tissue samples are not readily available on a fee-for-service basis. In a research study using experimentally infected mice, the PCR assay had higher sensitivity than microscopic methods in detecting parasitemia when evaluating animals over the course of infection.119 In a research study using experimentally infected dogs, parasite DNA was easily detected in the acute phase of infection in 12 animals, but the sensitivity of the test decreased during the chronic phase of infection depending on the strain of parasite.220 In a second experimental study, the PCR assay also showed a variable sensitivity rate, which increased as multiple sequential samples were evaluated during the course of disease.10 Isolation of organisms through the use of in vitro culture systems is a sensitive but time-consuming practice. Several culture methods have been described. In an experimental system, inoculation of mouse blood onto blood agar slants overlaid with liver infusion tryptose, medium or liver infusion tryptose alone was effective for isolating trypomastigotes from an infected animal, but 2 to 20 weeks may be needed for the results to become positive for epimastigotes.29,128 Direct inoculation of blood into a Vero cell monolayer will usually result in the development of intracellular amastigote forms and trypomastigotes in media after 2 to 4 weeks.29 In one naturally infected dog, parasite cultures were established using lymph-node aspirates inoculated into serum-supplemented Dulbecco’s modified Eagle’s medium and maintained at room temperature to yield replication of the flagellated epimastigote stage of parasite.158 Xenodiagnostic methods of inoculating blood into mice, or using bug feeding on suspected infected hosts, to detect parasite infections have been described. These methods are labor intensive, show variable sensitivity, and do not provide rapid results. Therefore, these methods are used primarily in a research setting and have limited application in making a clinical diagnosis. Although specificity of bug feeding is high, the sensitivity may be low; results of one study were only an 11% detection rate in bugs fed serially on experimentally infected dogs.10 In dogs that succumb to acute disease, lesions are usually confined to the heart, and sometimes abnormalities of the right atrium and ventricle are more severe than those of the left chambers.195 Subendocardial and subepicardial hemorrhages, as well as multiple yellow to white myocardial spots and streaks, have been reported in natural and experimental infections.27,205 Generalized lymphadenomegaly may also be noted.27,29,29 Additionally, in experimental infections, hepatic, splenic, and renal congestion, as well as pulmonary edema, have been reported secondary to cardiac failure.27 Chronic disease is characterized by a bilaterally enlarged flaccid heart with areas of thinning of the ventricular walls. Serosanguineous pleural and abdominal effusions have also been reported in naturally infected dogs.28 Microscopically, multifocal to diffuse granulomatous myocarditis with myocardial necrosis is characteristic in acute infections.4,205 Numerous pseudocysts containing amastigotes are often associated with the inflammatory response (Fig. 72-6). Typically, the heart is the primary organ with significant lesions, but amastigotes can be identified in enlarged lymph nodes and sometimes in other organs.158 In experimental infections in young dogs, mild granulomatous myositis has been observed, and organisms have been found in the smooth muscle of the stomach, small intestine, bladder, and skeletal muscle as well as the heart.25 Nonsuppurative encephalitis was also found in several of the dogs.25 In another naturally infected dog, myositis with parasites was identified in the diaphragm, and pulmonary congestion and edema were found, in addition to cardiac lesions.195
Trypanosomiasis
American Trypanosomiasis
Etiology and Life Cycle
Epidemiology
Trypanosoma cruzi Infection
Location
Year
Status
Assay
No. of Positive/Total Tested (% Positive)
Reference
UNITED STATES
Tennessee
2009
Pet
Indirect FA
55/860 (6.4)
193
35 states in the United States and Canada
2006
Pet
RIPA
86/413 (21.0)
71
Houston, TX
2004
Pet
ELISA
53/356 (14.9)
203
FC ALTA
5/356 (1.4)
Cameron County, TX
2003
Stray
Indirect FA
28/375 (7.5)
31
Oklahoma
2000
Pet and stray
RIPA
11/301 (3.7)
37
Louisiana
1991
Pet
ELISA
4/85 (4.7)
24
Stray
4/176 (2.3)
Seven southeastern statesa
1981
Pet
DA
24/365 (6.6)
214
LATIN AMERICA
Mexico
2006
Pet
ELISA/IFC
24/114 (21.0)
75
Argentina
2006
Pet
ELISA/Indirect FA/Indirect HA
41/68 (60.3)
93
Venezuela
2006
Pet
ELISA/Indirect FA/DA
382/565 (67.6)
55
Costa Rica
2002
Pet
ELISA/Indirect FA/Indirect HA
15/54 (27.7)
151
Brazil
1978
Pet
CF or indirect FA
5/27 (18.5)
156
Trypanosoma evansi Infection
Trypanosoma caninum species novum Infection
Pathogenesis
Clinical Findings
Dogs
Cats
Diagnosis
Electrocardiographic Findings
Medical Imaging Findings
Clinical Laboratory Findings
Cytologic Findings
Antibody Detection
Molecular Genetic Testing
Parasite Isolation and Xenodiagnosis
Pathologic Findings
Gross Lesions
Histopathologic Lesions
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Trypanosomiasis