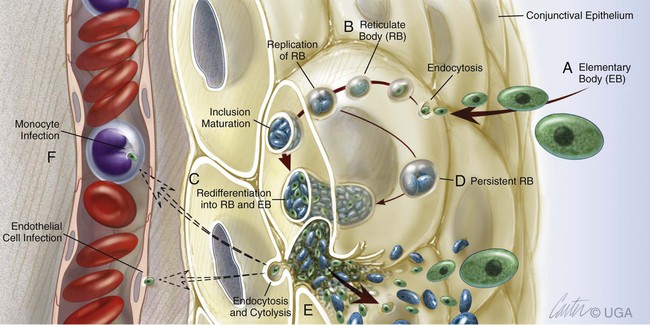
The genus Chlamydophila is a member of the order Chlamydiales and family Chlamydiaceae. Chlamydiae are obligate, generally gram-negative, intracellular parasites that, like bacteria, have a cell wall, DNA, and RNA but lack the metabolic machinery required for autonomous survival and replication. They multiply by binary fission within membrane-bound cytoplasmic vacuoles of host cells. They have an unusual developmental cycle that involves both extracellular (elementary body) and intracellular (reticulate body) forms (Fig. 28-1). The family Chlamydiaceae has been divided into two genera, Chlamydia and Chlamydophila (Table 28-1).26 Species within the genera are roughly host-associated, although evidence for an expanded host range for different species is increasing. The genus Chlamydia contains Chlamydia trachomatis, which causes chronic ocular disease (trachoma) and urogenital disease in humans; Chlamydia muridarum, which infects rodents; and Chlamydia suis, which infects swine and sheep.74 The genus Chlamydophila (Cp.) contains several species of medical and veterinary importance, including Cp. psittaci, Cp. pneumoniae, Cp. pecorum, Cp. abortus, Cp. Suis, Cp. caviae, and Cp. felis.26 Cp. psittaci is the type species for this genus and is the causative agent of psittacosis in birds and humans; it has recently also been associated with trachoma in humans.12 Cp. pneumoniae is a human pathogen producing respiratory infections and has also been linked to coronary artery disease, asthma, cerebrovascular disease, sarcoidosis, trachoma and reactive arthritis. This organism is also associated with ocular adnexal mucosal-associated lymphoid tissue lymphoma in humans.28 Cp. pecorum infects cattle and sheep. Cp. abortus infects sheep and has also been identified in a dog; Cp. caviae infects guinea pigs and was identified in a dog; and Cp. felis is the predominant organism infecting cats and has been identified in dogs.73 TABLE 28-1 Comparison of Classification of Chlamydophila and Chlamydia In addition to genetic differences, the different chlamydial species can be differentiated phenotypically by their monoclonal antibody (MAB) reactivity, and to a certain extent, morphologically and by their staining properties. The ultrastructural morphologies of Cp. psittaci and C. trachomatis are virtually identical, but Cp. pneumoniae forms pear-shaped elementary bodies.30 C. trachomatis forms compact rigid cell wall inclusions, containing glycogen, that stain with iodine, a characteristic that distinguishes it from Cp. psittaci. Generally, each chlamydial species has a preferred host range, often of a few or single host animal species, sometimes producing respiratory, genital, and systemic illness. The complete genome sequence of Cp. felis has been elucidated.2 The genome contains an 1166-kb circular chromosome, encoding 1005 protein-coding genes, and a 7.5-kb circular plasmid. Based on sequence analysis of the chlamydial outer membrane protein gene (ompA), isolates from cats worldwide are genetically similar. Conversely, random amplification of polymorphic DNA (RAPD) fingerprinting has revealed at least two subtypes.8,77,77 Serologic relationships may be more diverse.49,76 Chlamydia-like organisms also exist as endosymbionts of free-living amebae. Amebae are ubiquitous in soil and environmental water sources and can be isolated commonly from human mucosal surfaces, occasionally causing keratitis and disseminated infections.86 The life cycle of the chlamydial endosymbionts resembles that of the chlamydiae, with the exception of the existence of a third developmental stage, the crescent body.35 The endosymbionts have been grouped within the family Parachlamydiaceae and include Neochlamydia, Parachlamydia, Protochlamydia, Rhabdochlamydia, Criblamydia, Simkania, and Waddlia spp. Neochlamydia hartmannellae has been detected using the polymerase chain reaction (PCR) in cats with ocular disease, and similar agents have been detected in the gills of Arctic Char with epitheliocystis.23 Parachlamydia acanthamoebae has been associated with pneumonia in humans, and the pulmonary pathogenicity of Protochlamydia naegleriophila, Simkania negevensis, and Waddlia chondrophila is under investigation.34 Parachlamydia species have been associated with abortion in cattle and small ruminants.82,83 The following discussion focuses on chlamydial infections of cats and dogs. Positive antibody titers to chlamydiae have been detected in as many as 11% of healthy cats43 and 45% of farm cats. Despite this high prevalence of seroreactivity, Chlamydophila was isolated from 6% of conjunctival swabs and 4% of rectal swabs from clinically healthy cats. Isolation rates of up to 30% of household cats with conjunctivitis were reported.106 In household cats, clinically healthy cats, those with a history of and presence of past conjunctivitis, and those with active conjunctivitis, positive PCR results were 0%, 4.6%, and 7.3%, respectively.55 In shelter cats, the prevalence of Cp. felis infection has ranged from 0%4,45,56,103 to 15%.4 In cats from European catteries, without or with ongoing clinical respiratory disease, the prevalence rates for infection were 3% and 10%, respectively.41 Serologic studies in cats coupled with organism culture or genetic detection indicate that the chance of exposure and increasing immunity are greater with advancing age.78,95,95 Cats 2 to 6 months of age are likely to be infected with Cp. felis, as are cats up to 1 year of age. Thereafter, the prevalence of infection decreases, and cats older than 5 years of age are very unlikely to be infected with Cp. felis. Cats less than 8 weeks of age are less likely to be infected, presumably from passive immunity from nursing. A primary mode of transmission of infection for neonatal kittens is thought to be from the genital mucosae of the dam during parturition. In older cats, transmission occurs from close direct or aerosol contact. Venereal transmission, as described in other hosts, has not yet been confirmed in cats. In epidemiologic surveys for respiratory disease, the prevalence of detection depends on the method used, geographic location, presence of clinical illness, and age of cats (Web Table 28-1). Cats over 1 year of age have progressively lower isolation rates as they continue to age, which likely relates to natural enhancement of immunity and clearing of infection with advancing age. Conjunctivitis is more likely associated with Chlamydophila infection, whereas sneezing is more likely in feline herpesvirus type 1 (FHV-1) infections.95 Chlamydophila prevalence rates have been higher in the summer months.95 WEB TABLE 28-1 Prevalence of Chlamydophila Infection in Naturally Infected Domestic Cats Based on Culture or Genetic Detection Methodsa FA, Fluorescent antibody; PCR, polymerase chain reaction; URTD, upper respiratory tract disease. aNumerous serosurveys show a higher prevalence of positive results, indicating that exposure to Chlamydophila infection in cats is higher than the actual infection rate. Non–Cp. felis chlamydial DNA has been detected in feline conjunctival specimens that was 99% homologous to the sequence of N. hartmannellae, an amebic endosymbiont.104 These chlamydiae were found in association with the obligate ameboid host Hartmannella vermiformis. This ameba has been identified as a cause of ocular surface infection in people and may have some role in feline ocular infections (see Chapter 92). The chlamydiae were not observed cytologically. Parachlamydia acanthamoebae and related Chlamydiales were found in corneal samples from cats with and without corneal disease that were not harboring amebae.80a Therefore, the organisms may persist in the eyes of cats in the absence of ameboid hosts. Detection of Chlamydia-like organisms in the tissues of other host species has depended on use of immunohistochemical and electron microscopic methods. Cats infected with Cp. felis develop upper respiratory signs, including fever, ocular discharge, and sneezing. The appearance of conjunctivitis parallels the ocular shedding of chlamydiae. The predominant feature is inflammation of the conjunctiva or nictitating membrane. After conjunctival inoculation, organisms can spread systemically to colonize many tissues, including the tonsil, lung, liver, spleen, GI tract, and kidney.59 Infection has been transmitted experimentally by blood transfusion, with subsequent development of systemic signs and conjunctivitis.99 Infections with Cp. felis often become chronic and insidious. Cats may become asymptomatic but harbor the organism in the conjunctiva for 2 months or longer. Organisms have been isolated from the conjunctiva for up to 215 days after experimental infection.71,105 Whether chronic disease is the result of repeated reinfection or the presence of persistent chlamydiae is unclear. Persistent chlamydiae in the form of unculturable, atypical reticulate bodies have been identified in the joints of people with reactive arthritis and the intestinal tracts of pigs infected with C. suis.75 Cp. pneumoniae has been shown to survive and multiply within neutrophils until they become apoptotic, after which the organism is engulfed by pulmonary macrophages, which secrete anti-inflammatory cytokines as part of the apoptotic clearance process. In this way, the organism ensures a silent propagation to the interior of macrophages.84 When infected at 4 to 6 months or older, most cats shed organisms from the reproductive tract within a week after ocular inoculation. Shedding from the reproductive tract has not been documented in kittens younger than 13 weeks, even though they shed organisms from ocular discharges.99 It is possible that reactivation of shedding may occur in late pregnancy. These factors suggest that the hormonal influence on reproductive tissues or immune system may be important for the replication of organisms in the reproductive tract. The intestinal tract is another potential site of persistent infection in cats, as has been seen in other host species. Co-infections with other agents such as feline calicivirus, FHV-1, Bordetella, or Mycoplasma may increase the clinical severity of infection and duration of shedding. Other bacteria, including those that normally colonize the healthy conjunctival sac, can also act as secondary invaders and worsen disease. The febrile response in chlamydiosis is associated with chlamydemia and an acute-phase reaction in which the release of interleukin (IL)-1, IL-6, tumor necrosis factor, and interferons occurs. Acute phase reactants and IL-6 are increased in the plasma of experimentally infected cats.99 Little is known about the course of chlamydial infection in dogs. Case reports exist that describe chlamydiae in dogs with keratoconjunctivitis, respiratory signs, reproductive failure, arthritis, atherosclerosis, and encephalitis. Chronic disease manifestations in the dog may reflect dissemination and an immune response to organisms persisting in deeper host tissues, as has been shown to occur with chlamydial infections of other host species (see the discussion on human infection under Public Health Considerations). Using immunohistochemistry with MABs and PCR, chlamydiae have been detected in atheromatous lesions of the aorta, coronary, and splenic arteries of dogs.85
Chlamydial Infections
Etiology
Hosts
Preferential Tissues
Species
CHLAMYDOPHILA
Birds, humans
Genital, lung, eye, internal organs
Chlamydophila psittaci
Cattle, sheep
Brain, eye, joints
Chlamydophila pecorum
People, koala, horses
Lung, joints, eye, endothelium
Chlamydophila pneumoniae
Sheep, mammals
Intestines, placenta, eye
Chlamydophila abortus
Guinea pigs
Bladder, eye, spleen
Chlamydophila caviae
Cats, humansa
Eye, genital, joints, lung
Chlamydophila felis
CHLAMYDIA
People
Ocular and urogenital, neonatal lung
Chlamydia trachomatis
Rodents
Many internal organs
Chlamydia muridarum
Swine, sheep
Eye, intestines, lung
Chlamydia suis
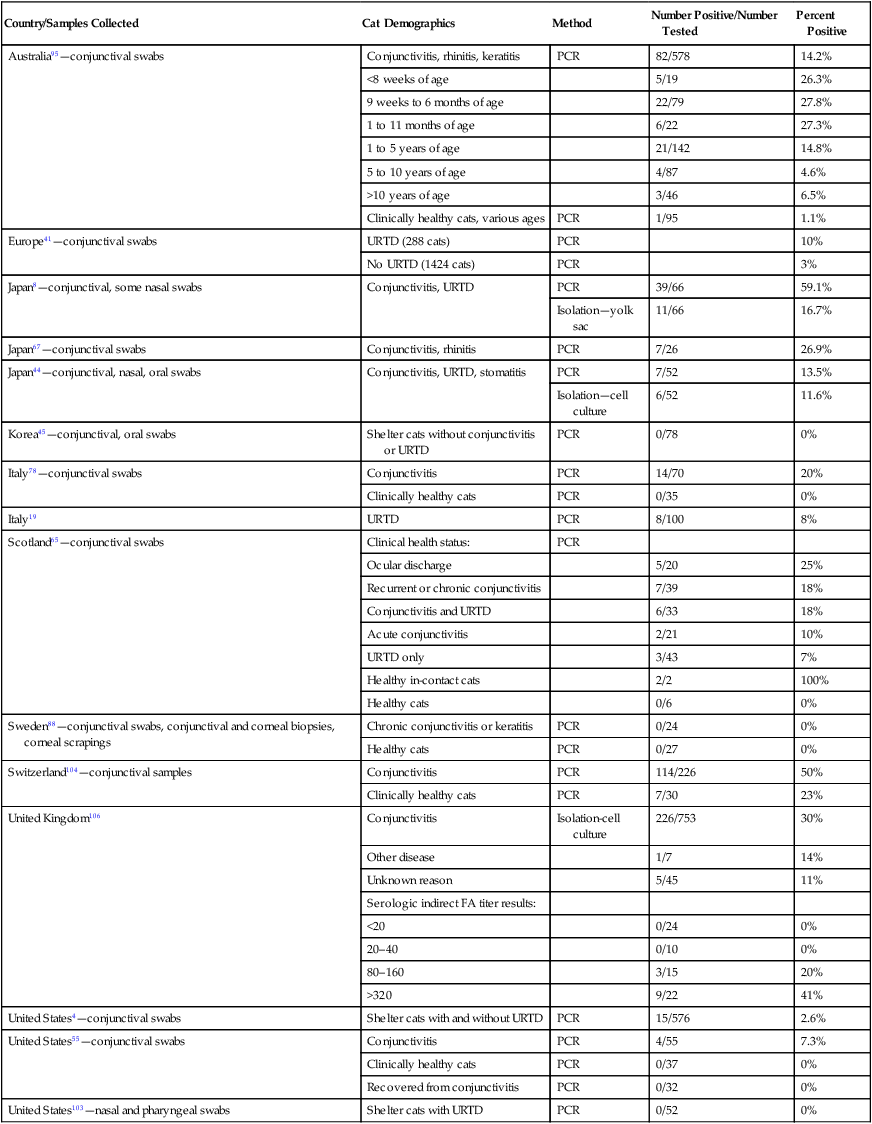
Epidemiology
Cats
Country/Samples Collected
Cat Demographics
Method
Number Positive/Number Tested
Percent Positive
Australia95—conjunctival swabs
Conjunctivitis, rhinitis, keratitis
PCR
82/578
14.2%
<8 weeks of age
5/19
26.3%
9 weeks to 6 months of age
22/79
27.8%
1 to 11 months of age
6/22
27.3%
1 to 5 years of age
21/142
14.8%
5 to 10 years of age
4/87
4.6%
>10 years of age
3/46
6.5%
Clinically healthy cats, various ages
PCR
1/95
1.1%
Europe41—conjunctival swabs
URTD (288 cats)
PCR
10%
No URTD (1424 cats)
PCR
3%
Japan8—conjunctival, some nasal swabs
Conjunctivitis, URTD
PCR
39/66
59.1%
Isolation—yolk sac
11/66
16.7%
Japan67—conjunctival swabs
Conjunctivitis, rhinitis
PCR
7/26
26.9%
Japan44—conjunctival, nasal, oral swabs
Conjunctivitis, URTD, stomatitis
PCR
7/52
13.5%
Isolation—cell culture
6/52
11.6%
Korea45—conjunctival, oral swabs
Shelter cats without conjunctivitis or URTD
PCR
0/78
0%
Italy78—conjunctival swabs
Conjunctivitis
PCR
14/70
20%
Clinically healthy cats
PCR
0/35
0%
Italy19
URTD
PCR
8/100
8%
Scotland65—conjunctival swabs
Clinical health status:
PCR
Ocular discharge
5/20
25%
Recurrent or chronic conjunctivitis
7/39
18%
Conjunctivitis and URTD
6/33
18%
Acute conjunctivitis
2/21
10%
URTD only
3/43
7%
Healthy in-contact cats
2/2
100%
Healthy cats
0/6
0%
Sweden88—conjunctival swabs, conjunctival and corneal biopsies, corneal scrapings
Chronic conjunctivitis or keratitis
PCR
0/24
0%
Healthy cats
PCR
0/27
0%
Switzerland104—conjunctival samples
Conjunctivitis
PCR
114/226
50%
Clinically healthy cats
PCR
7/30
23%
United Kingdom106
Conjunctivitis
Isolation-cell culture
226/753
30%
Other disease
1/7
14%
Unknown reason
5/45
11%
Serologic indirect FA titer results:
<20
0/24
0%
20–40
0/10
0%
80–160
3/15
20%
>320
9/22
41%
United States4—conjunctival swabs
Shelter cats with and without URTD
PCR
15/576
2.6%
United States55—conjunctival swabs
Conjunctivitis
PCR
4/55
7.3%
Clinically healthy cats
PCR
0/37
0%
Recovered from conjunctivitis
PCR
0/32
0%
United States103—nasal and pharyngeal swabs
Shelter cats with URTD
PCR
0/52
0%
Pathogenesis
Cats
Dogs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine