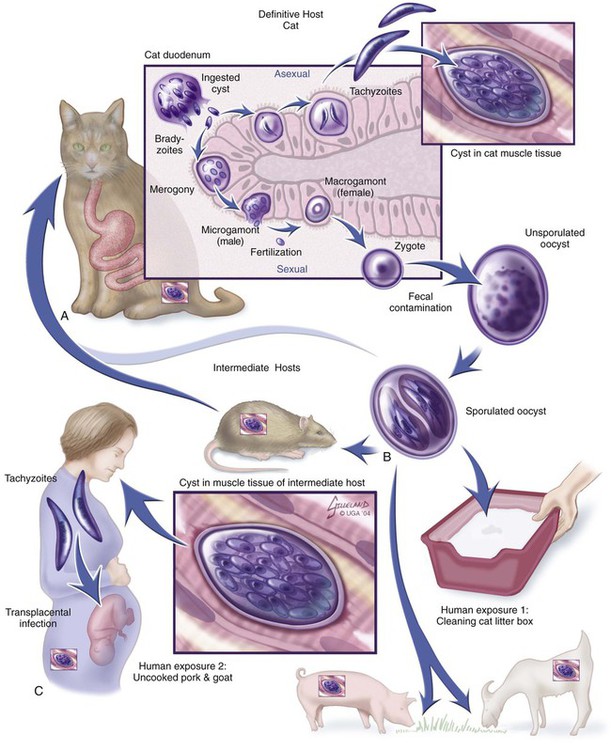
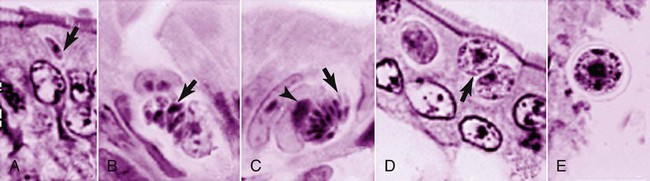
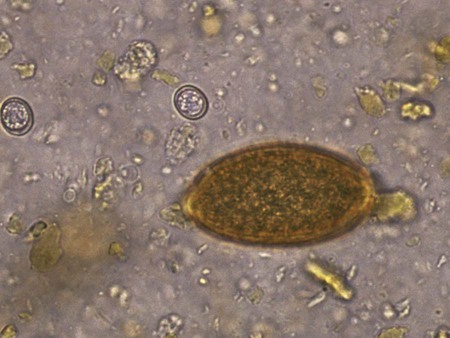
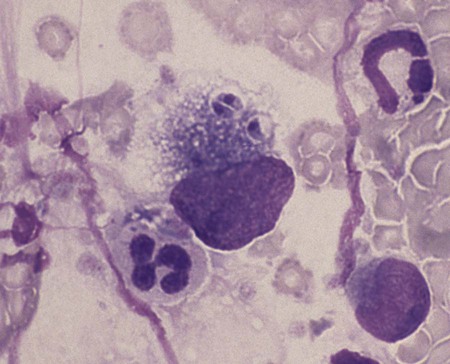
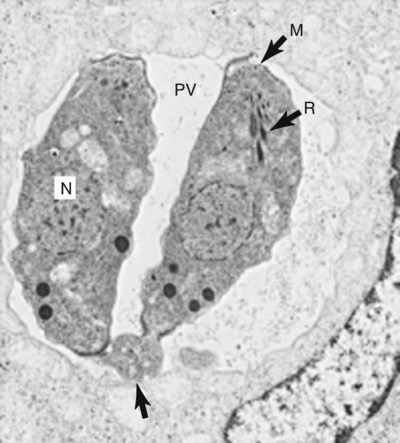
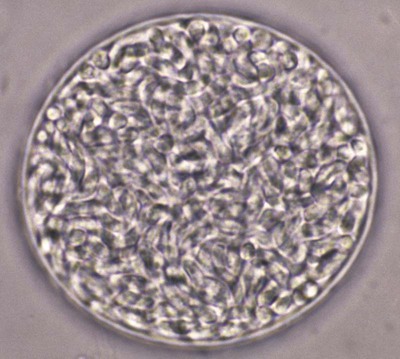
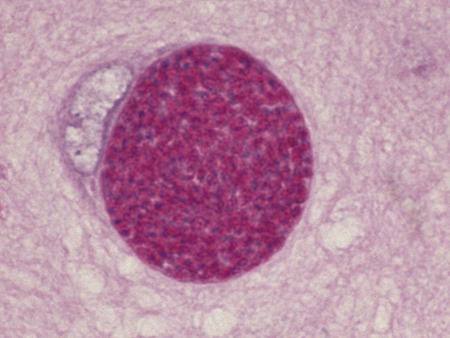
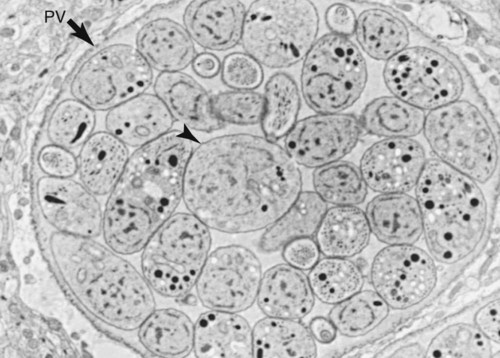
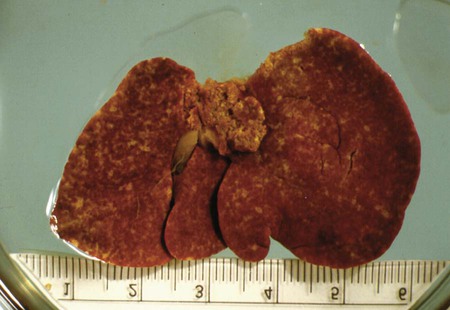
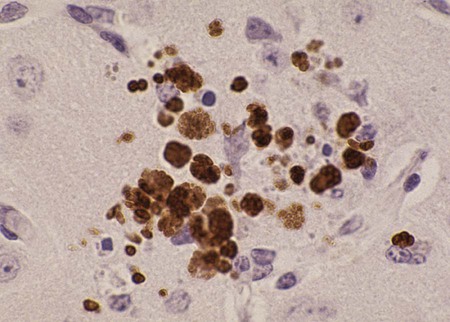
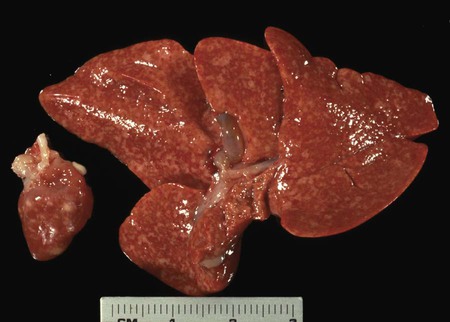
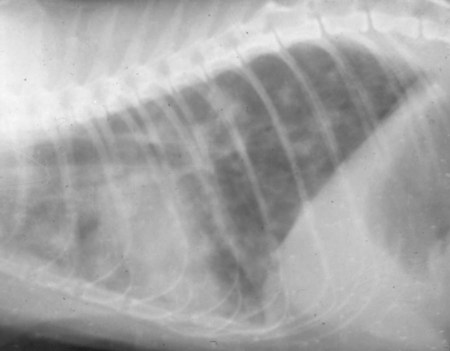
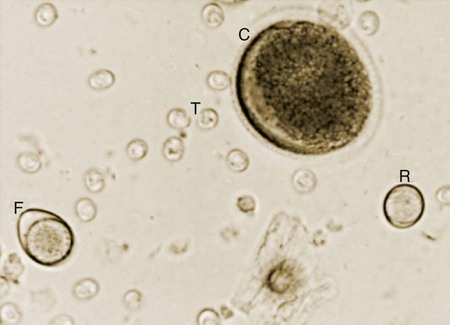
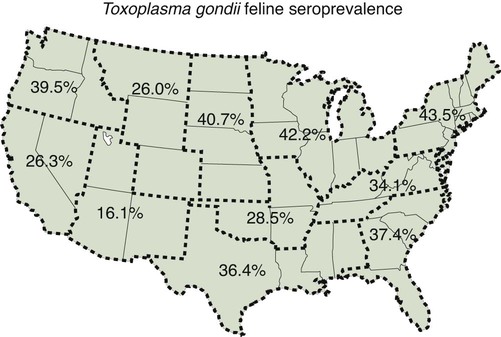
Toxoplasma gondii is an obligate intracellular coccidian parasite that infects virtually all species of warm-blooded animals, including people.145,147,147 Domestic cats and other Felidae are the definitive hosts that excrete oocysts. All nonfeline hosts are intermediate hosts that harbor tissue cysts. Three infectious stages have been noted: sporozoites in oocysts, tachyzoites (actively multiplying stage), and bradyzoites (slowly multiplying stage) enclosed in tissue cysts. Oocysts are excreted in feces, whereas tachyzoites and bradyzoites are found in tissues. Most isolates of T. gondii can be grouped into three genetic lineages that can be used in epidemiologic monitoring. The three predominant genotypes likely resulted from a single cross between two parenteral strains approximately 10,000 years ago.188,523 Greater genetic diversity has been observed among T. gondii isolates, especially isolates from Brazil, and severe clinical toxoplasmosis in immunocompetent humans has been reported associated with atypical isolates of T. gondii.1,145,145 Three main genotypes are responsible for most human infections. Type I isolates cause ocular disease in immunocompetent people and congenital infections. Type II isolates are associated with most infections of immunocompromised humans. Type I and type III infections have been found in the brains of dogs with neurologic dysfunction.100 Type II and type III are often found in animal infections.43a The three major modes of transmission are congenital infection, ingestion of infected tissues, and ingestion of oocyst-contaminated food or water (Fig. 79-1). Other minor modes of transmission include lactational,456 transfusion of body fluids, or transplantation of tissues or organs.44,147 T. gondii DNA has been found in Ixodes ricinus ticks518; however, the significance of vectors in transmission is unknown and may be secondary to their feeding on infected hosts. Toxoplasmosis and seroreactivity to T. gondii are more prevalent in older animals as a factor of increasing exposure with aging and in animals from a rural or feral environment that are more apt to hunt small mammals.414,476a A higher frequency of disease and exposure is found in dogs or cats that are fed raw meat instead of commercial diets.215,216,216 Seroprevalence is highest in older cats and in those that are kept outdoors, are homeless, or are found in shelters.* Outdoor cats in a Bangkok metropolitan area had a relatively low infection rate, presumably because they were predominantly fed well-cooked fish and rice.525 Toxoplasma infections are important in wild canine and feline hosts.† Free-living and impounded wild canids and felids have developed illness.302,511 Exotic carnivores in zoologic gardens have a high prevalence of infection, presumably from eating raw meat diets.362,500,500 Evidence has accumulated that toxoplasmosis is an important water-borne disease.138,285 Outbreaks have been associated with contamination of drinking-water supplies and natural fresh-water sources.121 In addition, T. gondii can survive in and sporulate in seawater and remain viable for at least 6 months at a temperature range of 4° C to 24° C.341,391 T. gondii has been identified as a cause of morbidity and mortality in marine mammals.206,535 Presumably, these animals become infected from runoff water that is contaminated with oocysts from cats and enters the ocean.138,390 Mollusks or marine invertebrates may act as transfer hosts. Oocysts from outdoor or indoor cats may enter the marine environment by entering storm-water drainage or sewage systems by being flushed from toilets and in open public drainage.188 This cycle is found only in the definitive feline host (see Fig. 79-1). Most cats are thought to become infected by ingesting intermediate hosts infected with T. gondii. Bradyzoites are released in the stomach and intestine from the tissue cysts when digestive enzymes dissolve the cyst wall. Bradyzoites penetrate the epithelial cells of small intestine and initiate the five types of predetermined asexual stages (Fig. 79-2). These types, A to E, are equivalent to schizonts of other intestinal coccidia.513 After an undetermined number of generations, merozoites released from type D or E form male (micro-) or female (macro-) gamonts, respectively. The microgamont divides and forms several biflagellate microgametes, which are released and swim to and penetrate macrogamonts.513 A wall is formed around the fertilized macrogamont to form an oocyst. Oocysts are round to oval, 10 µm × 12 µm, and are unsporulated (uninfective) when passed in feces (Fig. 79-3). After exposure to air and moisture for 1 to 5 days, oocysts sporulate and contain two sporocysts, each with four sporozoites. Sporozoites are banana-shaped, approximately 8 µm × 2 µm, and can survive in the oocyst for many months, even under harsh environmental conditions. These sporozoites are the infectious form of the oocyst. The entire enteroepithelial (coccidian) cycle of T. gondii can be completed within 3 to 10 days after ingestion of tissue cysts and occurs in up to 97% of naïve cats. However, after ingestion of oocysts or tachyzoites, the formation of oocysts is delayed until 18 or more days, and only 20% of cats fed oocysts will develop patency.127,129,129 The differences in the life cycle that account for this delay and resistance are uncertain, but bradyzoites are probably precursors of enteroepithelial replication, allowing for more rapid development. Bradyzoites are more infective to cats as compared with oocysts because many fewer of the former are needed to establish patent infections.1,140 Dogs have been infected by feeding on tissue cysts.345 Experimentally, dogs have been fed sporulated oocysts from cat feces, which passed through into their feces for 2 days postinoculation (PI).346 Although these dogs seroconverted, no clinical signs of infection or enteroepithelial replication occurred. Dogs ingesting cat litter would serve as potential mechanical vectors for transmission to humans as they shed ingested sporulated oocysts in their stool.346 The extraintestinal development of T. gondii is the same as it is for all hosts, including rodents, dogs, cats, and humans, and is not dependent on whether tissue cysts or oocysts are ingested. After the ingestion of oocysts, sporozoites excyst in the lumen of the small intestine and penetrate intestinal cells, including the cells in the lamina propria. Sporozoites divide into two by an asexual process known as endodyogeny and thus become tachyzoites. Tachyzoites are lunate in shape, approximately 6 µm × 2 µm (Figs. 79-4 and 79-5) and multiply in almost any cell of the body. If the cell ruptures, they infect new cells. Otherwise, tachyzoites multiply intracellularly for an undetermined period and eventually encyst. Tissue cysts grow intracellularly and contain numerous bradyzoites (Figs. 79-6, 79-7, and 79-8). Bradyzoites resemble tachyzoites in structure except that they are thinner and the nucleus is located at the posterior (nonconoidal) end of the parasite. Biologically, bradyzoites differ from tachyzoites in that they can survive the digestive process in the stomach, whereas tachyzoites are usually killed. Tissue cysts vary in size from 15 to 60 µm and usually conform to the shape of the parasitized cell. Tissue cysts are separated from the host cell by a thin (less than 0.5 µm) elastic wall (see Fig. 79-8). Tissue cysts are formed in the central nervous system (CNS), muscles, and visceral organs and probably persist for the life of the host. Some variation in tissue tropism for cyst formation has been observed.129 Organisms encysted with Toxoplasma can injure the CNS of infected mice and rats, resulting in impaired learning and memory and behavioral abnormalities. T. gondii–infected rodents become less neophobic, leading to decreased aversion to the odor of cats.42,561 This behavior might help ensure that rodents would be eaten by cats and the life cycle would be preserved; however, the neurologic signs were transient and corresponded with peak development of tissue cysts in the brain.273 Wild house mice (Mus domesticus) and field mice (Apodemus sylvaticus) have been shown to transmit the infection congenitally at a rate of 75% or greater.370,430 This may serve as a mechanism of maintaining the infection in nature in some rodents. Parasitemia during pregnancy can cause placentitis followed by spread of tachyzoites to the fetus. In humans or sheep, congenital transmission usually occurs when the woman or ewe becomes initially infected during pregnancy. Bitches fed sporulated oocysts at 56, 40, and 32 days of gestation showed evidence of congenital infection and aborted, but these results need confirmation; confirmed reports of congenital toxoplasmosis in dogs are rare.10,55,55 Many kittens born to queens infected with T. gondii during gestation became infected transplacentally or via suckling as tachyzoites are shed in milk.* Clinical illness was common, varying with the stage of gestation at the time of infection, and some newborn kittens shed oocysts.173 Lactational or transplacental transmission may predispose kittens to the development of ocular toxoplasmosis.458 The type and severity of clinical illness with T. gondii infections depend on the degree and localization of tissue injury. Tachyzoites are the invasive asexual forms of the parasite that require intracellular existence for replication and survival. All cell types appear susceptible. Cell necrosis is caused by the intracellular growth of Toxoplasma. T. gondii does not produce a toxin. In infections acquired after the ingestion of tissue cysts or oocysts, initial clinical signs are caused by the necrosis of intestine and associated lymphoid organs caused by tachyzoites. Enteroepithelial stages in the cat appear to have unique antigens.530 Antibodies of the IgA class, specific for T. gondii enteroepithelial stages, increase in the intestinal secretions as part of the immune response to terminate the phase of intestinal replication.424,481 T. gondii also spreads to extraintestinal organs via blood or lymph, and focal necrosis may develop in many organs (Fig. 79-9). The brain, liver, lungs, skeletal muscle, and eyes are common sites of initial replication and chronic persistence of infection. The clinical outcome is determined by the extent of injury to these organs, especially vital organs such as the heart, lung, liver, and adrenal glands. Although acute disseminated infections can be fatal, the host often recovers. Interferon (IFN)-γ-dependent cell-mediated immunity and humoral factors are important in resistance to Toxoplasma encephalitis as studied in experimentally infected rodents.527 Resistance is caused by interplay among T and B lymphocytes, IFN-γ-producing non-T cells, microglia, astrocytes, and dendritic cells. The classical complement pathway of cats is not activated after exposure to T. gondii,290 a feature that appears unique to the definitive host. By approximately the third week after infection, tachyzoites begin to disappear from visceral tissues and may localize as tissue cysts (as bradyzoites). This phase is associated with a systemic immune response, which inhibits the parasitemia. These tissue cysts may persist in the host for life (Fig. 79-10). Tissue cysts may rupture, and released bradyzoites may initiate a clinical relapse during immunosuppression, such as with antitumor or glucocorticoid therapy. The mechanism of reactivation is not known. The reason why some infected dogs or cats develop clinical toxoplasmosis while others remain well is not fully understood. Age, sex, host species, strain of T. gondii, number of organisms, and stage of the parasite ingested may account for some of the differences. Postnatally acquired toxoplasmosis is generally less serious than is prenatally acquired infection. Stress may also aggravate T. gondii infection. Concomitant illness or immunosuppression may make a host more susceptible because T. gondii proliferates as an opportunistic pathogen. Clinical toxoplasmosis in dogs is often associated with canine distemper or other infections, such as ehrlichiosis, or with glucocorticoid therapy or vaccination with live attenuated vaccines.107,154,154 In some cases, however, predisposing disorders cannot be found. Historically, the prevalence of canine toxoplasmosis has decreased with the routine use of canine distemper vaccines.154 Some cases of clinical feline toxoplasmosis have been observed concomitantly with glucocorticoid or ciclosporin therapy, hematotropic mycoplasmal infection (hemobartonellosis), feline leukemia virus (FeLV) and immunodeficiency virus (FIV) infections, and feline infectious peritonitis.* In one serosurvey of urban stray cats, cats infected with FIV were more likely to have seropositive test results for T. gondii, whereas no association with concurrent FeLV infection was found.120 A study reviewed all reports of concomitant infections in cats and concluded that concurrent infections with Bartonella spp., FIV, or FeLV did not affect the magnitude of titer and seroprevalence of T. gondii in asymptomatic cats.149 More severe Toxoplasma infection occurs in cats that are co-infected with FIV.5a,104,320 FIV-infected cats that were subsequently co-infected with T. gondii had suppressed CD4+ and CD8+ lymphocyte expression of interleukin (IL)-2, IL-6, IFN-γ, and IL-12 when compared with cats infected with T. gondii alone.333 IL-10 levels were increased in FIV-infected cats before and after infection with T. gondii and may have suppressed the T1 helper cytokine response to T. gondii infection. In contrast to these experiments, experimental FIV and FeLV infections in cats did not produce reactivated or more severe acute infections in cats already or simultaneously inoculated with T. gondii.317,322,322 Regardless of the difference in these experimental studies or which infection precedes the other, clinically, cats with FIV and T. gondii co-infections are more difficult to treat effectively (see sections to follow). IL-6 activity is greatly elevated in cats with uveitis suspected to be a result of their Toxoplasma infection,319 and cats with toxoplasmosis have circulating T. gondii–containing immune complexes323 that may play a role in development of ocular toxoplasmosis. Feline herpesvirus 1 and Bartonella henselae co-infections failed to activate ocular toxoplasmosis in congenitally infected kittens.457 Toxoplasmosis has been associated with dissemination and mortality in cats and dogs receiving immunosuppression with ciclosporin.2,44,44 However, in one study, administration of ciclosporin at 7.5 mg/kg, orally (PO), daily did not induce clinical illness or repeat oocyst shedding in cats experimentally infected with T. gondii 42 days earlier.311 In contrast, cats that have ciclosporin trough levels that are above the normal range can develop disseminated disease when exposed to T. gondii for the first time. Thus, T. gondii–seronegative cats administered ciclosporin should not be fed raw meat or be allowed to hunt and should have blood levels measured and the dose adjusted to stay within therapeutic ranges. Naïve cats ingesting bradyzoites from tissues can develop self-limiting, small-bowel diarrhea as a manifestation of enteroepithelial replication. Otherwise, this phase of the illness, which lasts up to 10 days, can be clinically silent and unimportant. Immunosuppressed cats can have simultaneous systemic manifestations with extraintestinal spread of tachyzoites. However, clinical toxoplasmosis from systemic spread is most severe in transplacentally or lactationally infected kittens because tachyzoite replication can be overwhelming.151,173,173 Affected kittens may be stillborn or may die before weaning. Kittens may continue to suckle until death. Clinical signs reflect inflammation of the liver, lungs, and CNS (Fig. 79-11). Lethargy, depression, hypothermia, and sudden death can occur. Affected kittens may have an enlarged abdomen because of enlarged liver and ascites. Encephalitic kittens may sleep most of the time or cry continuously. With spinal cord involvement, paresis and ataxia, or paralysis has been observed.367 Kittens born to infected queens develop chorioretinitis, sometimes in the absence of other signs of clinical illness.458 Some kittens develop transient concurrent anterior uveitis. Rare reports of disseminated toxoplasmosis occur in old cats.514a Clinical signs in older cats can be the result of spread of tachyzoites after initial acute exposure or reactivation of a chronic encysted infection by release of bradyzoites after immunosuppression. Anorexia, lethargy, and dyspnea caused by pneumonia have been commonly recognized features of postnatal toxoplasmosis (Box 79-1). Other clinical findings include persistent or intermittent fever, anorexia, weight loss, icterus caused by hepatitis or cholangiohepatitis, myocarditis, vomiting, diarrhea, abdominal effusion, hyperesthesia on muscle palpation, stiffness of gait, shifting-leg lameness, neurologic deficits, cystitis, dermatitis, and death.* In 100 cats with histologically confirmed toxoplasmosis, clinical syndromes were diverse, but infection of pulmonary (97.7%), CNS (96.4%), hepatic (93.3%), pancreatic (84.4%), cardiac (86.4%), and ocular (81.5%) tissues were most common.150 Vomiting can be caused by lesions in parenchymal organs or in the walls of the gastrointestinal (GI) tract.379 Clinical signs may be sudden or have a slow onset. The disease may be rapidly fatal in some cats with severe respiratory or CNS signs. Anterior or posterior uveitis involving one or both eyes is common.† Iritis, iridocyclitis, or chorioretinitis can occur alone or concomitantly. Aqueous flare, keratic precipitate, lens luxation, glaucoma, and retinal detachment are common manifestations of uveitis. Chorioretinitis, which can be unifocal or multifocal, may occur in both tapetal and nontapetal areas (Fig. 79-12) (see Toxoplasmosis in Cats and Dogs, Chapter 92). Ocular toxoplasmosis occurs in some cats without polysystemic clinical signs of disease. In experimental T. gondii in cats, those infected concurrently with FIV developed severe pneumonitis and hepatitis, whereas those not infected with FIV developed multifocal chorioretinitis and anterior uveitis.102,104 Neurologic and ocular manifestations that occur in the absence of other systemic signs are more common with reactivated infection rather than acute infection. Although reactivated infections can occur anywhere in the nervous system, segmental myelitis has been a common finding in cats11a,158,258,354a and is characterized by hemi-, tetra-, or para-, paresis or paralysis. Reflexes are upper- or lower-motor neuron in nature depending on the level of spinal cord involvement. Electrocardiographic changes, characteristic of myocarditis, were observed in a cat with suspected toxoplasmosis.503 Nodular pyogranulomatous dermatitis has developed in cats with disseminated infection, usually resulting from immunosuppressive drug therapy.13,329,355,434 Clinical signs may be localized in respiratory, neuromuscular, or GI systems, or they may be caused by generalized infection.147,154 The neurologic form of toxoplasmosis may last for several weeks without involvement of other systems, whereas severe disease involving the lungs and liver may kill dogs within a week.147 Generalized toxoplasmosis is seen mostly in dogs younger than 1 year and is characterized by fever, tonsillitis, dyspnea, diarrhea, and vomiting. Icterus usually results from extensive hepatic necrosis.154,155 Myocardial involvement is usually subclinical, although arrhythmias and heart failure may develop as predominant findings in some dogs. As in cats, occasionally, there is dermatitis in dogs that are immunosuppressed.560 The most dramatic clinical signs in older dogs have been associated with neural and muscular systems.252 Neurologic signs depend on the site of lesion in the cerebrum, cerebellum, or spinal cord. Seizures, cranial nerve deficits, tremors, ataxia, and paresis or paralysis may be seen. Dogs with myositis may initially show abnormal gait, muscle wasting, or stiffness. Paraparesis and tetraparesis may rapidly progress to lower motor neuron (LMN) paralysis. In dogs with polyradiculoneuritis, the prevalence of antibody reactivity to T. gondii was greater (55.8%) than in nonaffected control dogs (11.4%).268a Dogs with this syndrome should be evaluated with toxoplasma antibody testing. Canine toxoplasmosis is clinically similar to Neospora caninum infection, which was previously confused with toxoplasmosis (see Neosporosis later in this chapter).439 Although these diseases are similar, toxoplasmosis appears to be more prevalent in cats and neosporosis more prevalent in dogs. Only a few reports have been submitted of ocular lesions associated with toxoplasmosis in dogs. Retinitis, anterior uveitis, iridocyclitis, ciliary epithelium hyperplasia, and optic nerve neuritis have been noted (see Toxoplasmosis in Cats and Dogs, Chapter 92). Keratoconjunctivitis was reported in a dog administered glucocorticoids topically.529 Routine hematologic and biochemical parameters may be abnormal in cats and dogs with acute systemic toxoplasmosis. Nonregenerative anemia, neutrophilic leukocytosis, lymphocytosis, monocytosis, and eosinophilia are most commonly observed. Leukopenia of severely affected cats may persist until death and is usually characterized by an absolute lymphopenia and neutropenia with an inappropriate left shift, eosinopenia, and monocytopenia. In experimentally infected cats, neutropenia and lymphopenia persist for 5 to 12 days. Leukocytosis was seen in the recovery phase of illness.322 Lymphocyte counts greater than 7000 cells/µL were common from 28 to 154 days after primary inoculation.322 Secondary exposure of cats to T. gondii did not result in significant changes in leukocyte numbers.322 Biochemical abnormalities during the acute phase of illness include hypoproteinemia and hypoalbuminemia. Hyperglobulinemia has been detected in some cats with chronic toxoplasmosis.325 Marked increases in serum alanine aminotransferase (ALT) and aspartate aminotransferase activities have been noted in animals with acute hepatic and muscle necrosis. Dogs generally have increased serum ALT and alkaline phosphatase activities with hepatic necrosis, but this occurs less frequently in cats. Serum creatine kinase (CK) activity is also increased in cases of muscle necrosis. Serum bilirubin levels have been increased in animals with acute hepatic necrosis, especially cats that develop cholangiohepatitis or hepatic lipidosis. Cats or dogs that develop pancreatitis may show increased serum amylase and lipase activities, although these are inconsistent. Cats often show proteinuria and bilirubinuria. Cats with pancreatitis may have reduced serum total calcium with normal serum albumin concentrations.150 However, it can be difficult to prove T. gondii–associated pancreatitis in cats. For example, in one study, there was no association between levels of feline pancreatic lipase immunoreactivity (fPLI) and T. gondii antibodies.36 Tachyzoites may be detected in various tissues and body fluids by cytology during acute illness (see Fig. 79-4). They are rarely found in blood, cerebrospinal fluid (CSF), fine-needle aspirates, and transtracheal or bronchoalveolar washings60,209,209 but are more common in the peritoneal and thoracic fluids of animals developing thoracic effusions or ascites. Polymerase chain reaction (PCR) can be performed to document that the agent is T. gondii. Inflammatory changes are usually noted in body fluids. In suspected feline toxoplasmosis of the nervous system, CSF protein levels were within reference ranges to a maximum of 149 mg/dL, and nucleated cells were a maximum of 28 cells/mL.325 Lymphocytes usually predominate, but a mixture of inflammatory cells may be found.410,505 Thoracic radiographic findings, especially in cats with acute disease, consist of a diffuse interstitial to alveolar pattern with a mottled lobar distribution (Fig. 79-13).485 Diffuse, symmetric homogeneous increased density caused by alveolar coalescence has been noted in severely affected animals. Mild pleural effusion can be present. Abdominal radiographic findings may consist of masses in the intestines or mesenteric lymph nodes or homogeneous increased density as a result of effusion. Loss of contrast in the right abdominal quadrant can indicate pancreatitis. Abdominal ultrasonographic changes can indicate organ or tissue enlargement as a result of inflammation or granuloma formation. Lesions within the CNS may be detected by myelography, computed tomography, or magnetic resonance imaging. Magnetic resonance imaging findings usually include multifocal lesions; however, solitary mass lesions (granulomas) may be observed.214,410,410 Despite the high prevalence of serum antibodies in cats worldwide, the prevalence of T. gondii oocysts in feces is low.285 Fewer than about 1% of cats shed oocysts on any given day.* Because cats usually shed T. gondii oocysts for only 1 to 2 weeks after their first exposure, oocysts are rarely found in routine fecal examination. Moreover, cats are usually not clinically ill and do not have diarrhea during the period of oocyst shedding.145 Although cats are considered immune to reshedding of oocysts, they may shed a few oocysts after rechallenge with different strains more than 6 years later.126 Cats that are immune have partial asexual development of T. gondii in their intestines compared with complete development cycle in naïve cats.105 Immunosuppression with a high dose (10 to 80 mg/kg, daily PO or intramuscularly once a week) of prednisolone will cause chronically infected cats to reexcrete oocysts, whereas a lower dose (5 mg/kg intramuscularly for 4 weeks) will not.318 Cats administered ciclosporin at 7.5 mg/kg, PO, daily for 42 days before experimental inoculation with T. gondii shed oocysts at a similar level and for a similar time length as cats inoculated with T. gondii without pretreatment.311 Cats with T. gondii infection that was initiated 42 days before initiation of ciclosporin treatment failed to have repeat T. gondii oocyst shedding when administered ciclosporin at 7.5 mg/kg, PO, daily for 42 days. Despite the low prevalence of oocyst shedding among cats, the absolute numbers of oocysts shed during their first exposure can have a major role in environmental contamination.98 T. gondii oocysts in feline feces are morphometrically indistinguishable from oocysts of Hammondia hammondi, Besnoitia oryctofelisi, and Besnoitia darlingi, which also occur in cats (see Chapter 80). Oocysts of these coccidians can be differentiated only by ultrastructural and molecular examination, sporulation, and subsequent animal inoculation.197,515 If 10-µm-sized oocysts are found, they should be considered to be T. gondii until proved otherwise. Because of the infectious nature of this organism, further inoculations should be attempted only in a diagnostic laboratory with competence in this procedure.145 Because of their small size, oocysts of T. gondii are best demonstrated by centrifugation using Sheather’s sugar solution. See Chapter 70 for a discussion of this technique. Examine 1 to 2 drops removed from the meniscus at low-power (×100) magnification. T. gondii oocysts are approximately one fourth the size of Isospora felis and one eighth the size of Toxocara cati (Fig. 79-14). A copro-PCR has been developed to increase the sensitivity of detection of T. gondii oocysts in the feces of cats.483a Once infected, animals harbor toxoplasmic tissue cysts for life, which stimulates a long-term humoral immune response in infected adults. Serologic surveys indicate that T. gondii infections are prevalent worldwide. Approximately 30% of cats and dogs in the United States have T. gondii antibodies (Fig. 79-15).110,147,285,552 The prevalence of seropositivity increases with age of the cat or dog because of the chance of exposure rather than susceptibility. Multiple serologic tests for the detection of antibodies have been used in the diagnosis of toxoplasmosis (Table 79-1). The use of these tests in cats has been reviewed.310 No single serologic assay exists that can definitively confirm a diagnosis of toxoplasmosis. The Sabin-Feldman dye test is highly sensitive and specific for human toxoplasmosis but not necessarily for feline infection. Moreover, the test is too technical to perform in diagnostic laboratories, and it uses live T. gondii. TABLE 79-1 Serologic Tests for Feline Toxoplasmosis ELISA, Enzyme-linked immunosorbent assay; FA, fluorescent antibody. For additional information on test availability, see Laboratory Listings, Web Appendix 5. aTiters (which are the reciprocal of serum dilutions) may vary between laboratories on the basis of individual methodologies. Whenever comparisons are being made, both samples should be processed at the same time by the same laboratory. The indirect fluorescent antibody (FA) technique is comparable to the dye test but does not require live antigen. Some false-positive polar staining that can occur with the indirect FA test has been attributed to Fc receptors on the surface of T. gondii tachyzoites that nonspecifically bind Ig. The indirect FA can be adapted to detect IgM, IgG, or IgA using whole or immunoblotted antigen (see later discussion).528 Agglutination tests have the advantage of being species independent and are available in commercial kits that have been developed for use in humans. The indirect hemagglutination (HA) test (TMP-test, Wampole Labs, Carter Wallace Inc., Cranbury, NJ) does not require live antigen but is less sensitive compared with the dye test, indirect FA test, and enzyme-linked immunosorbent assay (ELISA).327 The main drawback is that it primarily measures IgG and is usually not positive during acute infection. The latex agglutination test (LAT) (Toxo-test, Eiken Chemical Co., Tokyo, Japan) is somewhat more sensitive for serologic screening; however, it cannot be used to distinguish immunoglobulin classes. The modified agglutination test (MAT) (Toxo-Screen DA, BioMérieux, Marcy-l’Etoile, France) detects only IgG but is extremely sensitive compared with the other available assays.356 The MAT has been improved in its sensitivity and specificity in distinguishing acute and chronic Toxoplasma infections in humans and cats by using acetone- or methanol-fixed and formalin-fixed trophozoites, respectively.174 Antibodies to acetone-fixed antigen are elevated only during acute (under 3 months) infection, whereas antibodies to formalin-fixed antigen may remain high for several years.203 For additional information on commercial laboratory testing and test kits, see Web Appendices 5 and 6, respectively. ELISA, with and without immunoblotting, has been adapted for the detection of IgM, IgG, and IgA class antibodies against T. gondii in feline sera.* ELISA methods are as sensitive as is indirect FA50 and more sensitive compared with LAT or indirect HA. As a result, ELISA may be less specific at lower dilutions of sera. Immunoblots of separated antigens, reacted with an animal’s sera and ELISA, can be used to identify specific target antigens and improve specificity. Comparison of antigen recognition patterns by serum from infected queens and their kittens by immunoblot aids in the diagnosis of neonatal toxoplasmosis.70 ELISA has also been compared with indirect FA and indirect HA tests in the kinetics of the humoral immune response in dogs experimentally infected with T. gondii.498 Specific IgM was detected from day 7 PI and began decreasing by day 27 PI, with the ELISA showing slightly earlier and longer detection of IgM. Specific IgG was detected from day 7 PI throughout the 62 days of the study. IgM may constitute an indicator of recent or active infection in dogs. An ELISA incorporating a recombinant-produced immunodominant surface antigen (SAG)1 and P30 was developed to measure antibodies in experimentally infected cats and in experimentally and naturally infected dogs.298,498,498 The antigen is stage specific, being secreted by tachyzoites, and might be of consideration in the diagnosis of active clinical infections.298 Use of recombinant SAG2 in ELISA allowed for discrete detection of experimentally infected cats with the same specificity as that for the LAT.274,275 The test has also been adapted to an immunochromatographic method.275 The SAG2 ELISA appears to be specific because no cross-reaction occurred between sera of mice infected with Neospora and those infected with Toxoplasma.298 In studies in humans, IgG binding avidity for Toxoplasma antigens is low soon after primary antigenic challenge.335 The IgG avidity increases during subsequent months of infection from chronic humoral immune stimulation. Assays that quantitate the degree of antibody avidity might be used as a single assay to discriminate between recently acquired and chronic encysted infections (see Toxoplasmosis in Chapter 99).69a
Toxoplasmosis and Neosporosis
Toxoplasma Gondii Infection
Etiology
Epidemiology
Enteroepithelial Life Cycle
Extraintestinal Life Cycle
Congenital Transmission
Pathogenesis
Clinical Findings
Cats
Dogs
Diagnosis
Clinical Laboratory Findings
Cytology
Radiology
Fecal Examination
Serologic Testing
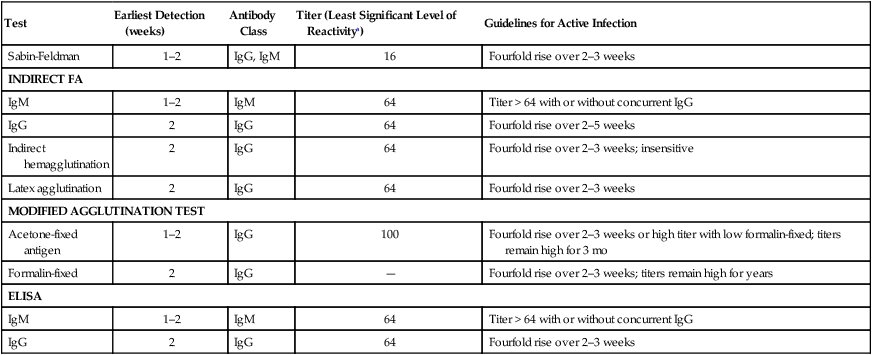
Test
Earliest Detection (weeks)
Antibody Class
Titer (Least Significant Level of Reactivitya)
Guidelines for Active Infection
Sabin-Feldman
1–2
IgG, IgM
16
Fourfold rise over 2–3 weeks
INDIRECT FA
IgM
1–2
IgM
64
Titer > 64 with or without concurrent IgG
IgG
2
IgG
64
Fourfold rise over 2–5 weeks
Indirect hemagglutination
2
IgG
64
Fourfold rise over 2–3 weeks; insensitive
Latex agglutination
2
IgG
64
Fourfold rise over 2–3 weeks
MODIFIED AGGLUTINATION TEST
Acetone-fixed antigen
1–2
IgG
100
Fourfold rise over 2–3 weeks or high titer with low formalin-fixed; titers remain high for 3 mo
Formalin-fixed
2
IgG
—
Fourfold rise over 2–3 weeks; titers remain high for years
ELISA
IgM
1–2
IgM
64
Titer > 64 with or without concurrent IgG
IgG
2
IgG
64
Fourfold rise over 2–3 weeks
Investigational Assays
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Toxoplasmosis and Neosporosis