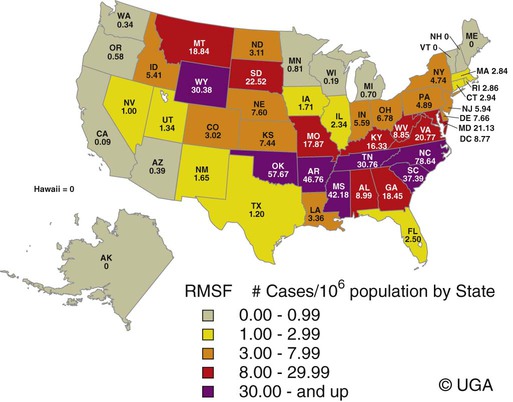
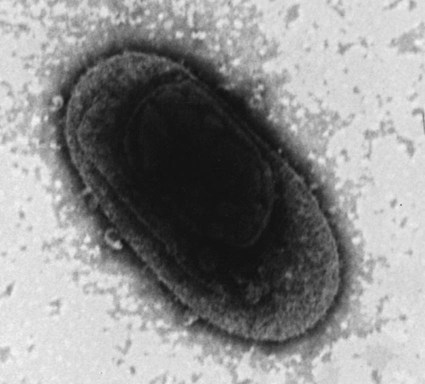
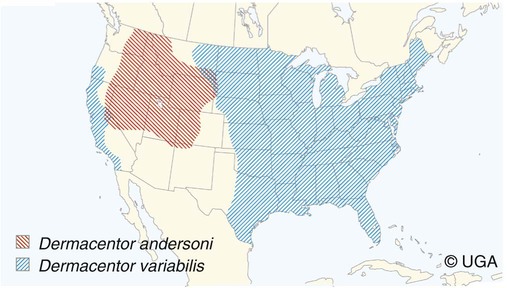
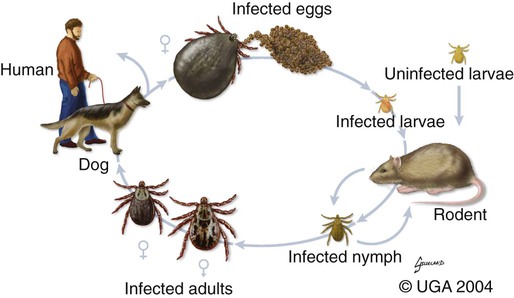
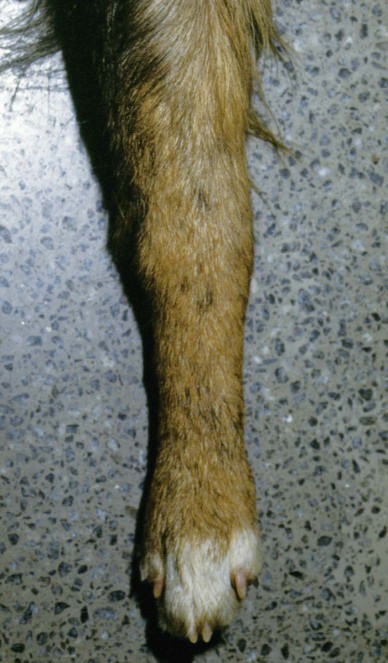
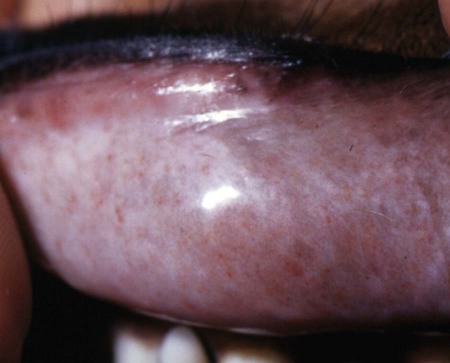
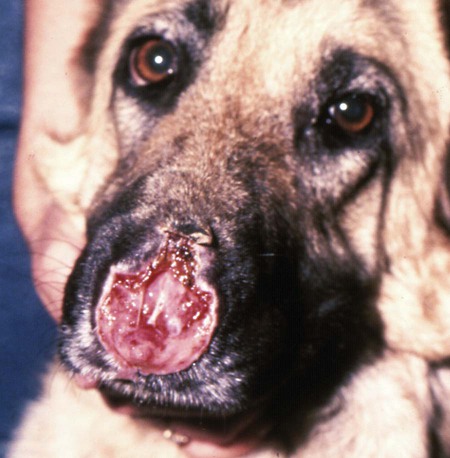
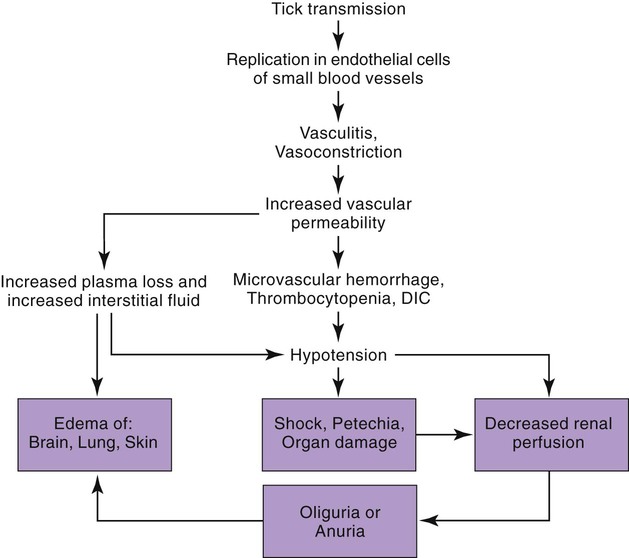
Rocky Mountain spotted fever (RMSF) is a tick-borne, rickettsial disease of the Americas that affects dogs and people. Although cats and other domestic animals can have serum antibodies that react with the causative agent, knowledge concerning the occurrence of disease in these other animals is minimal.137 RMSF was first recognized in the western United States before 1930. RMSF is now known to occur throughout the contiguous United States with the exception of Maine. Overall, the reported prevalence of human disease appears to have increased since its discovery; the highest annual incidence is now reported to be from the mid-Atlantic and eastern United States and some mid-southern states (Oklahoma and Arkansas) (Fig. 27-1). This increase may reflect better recognition and reporting in addition to geographic spread of the disease. Outside the United States, the disease has been reported in people living in western Canada, Mexico, Panama, Costa Rica, Honduras, Nicaragua, Colombia, Argentina, and Brazil.65 Deciduous forests, increased humidity, and warmer temperatures are factors associated with the high prevalence of this tick-transmitted disease in these areas. Rickettsia rickettsii, the etiologic agent of RMSF, is a member of order Rickettsiales and the family Rickettsiaceae (Fig. 27-2). The family Rickettsiaceae currently consists of two genera; Orientia and Rickettsia. Members of the genus Rickettsia were previously divided into the Spotted Fever Group (SFG), the Typhus Group, and the Scrub Typhus Group based on phenotypic criteria. Genetic analysis has resulted in the elimination of the Scrub Typhus Group. The sole member of this group, Rickettsia tsutsugamushi, now belongs in the genus Orientia. A combination of phenotypic and genotypic characteristics are now used for taxonomic classification of Rickettsia, although criteria for group and species determination are still evolving.176 Currently, the genus Rickettsia is divided into the SFG and Typhus groups (Table 27-1). Although controversial, the addition of a Transitional Group and an Ancestral Group based on genotypic criteria has been proposed.69 TABLE 27-1 Comparison of Some Vector-Borne Organisms of the Order Rickettsiales Affecting Dogs and Catsa aFor ehrlichiosis and other related rickettsiae, see Table 26-1. For neorickettsiosis, see Chapter 25. bAlso includes Spotted Fever Group (SFG)—rickettsiae causing recognized human illnesses: Japanese spotted fever (Rickettsia japonica: Japan), Queensland tick typhus (Rickettsia australis: Queensland and New South Wales, Australia), North Asian tick typhus (Rickettsia sibirica: Siberia, Mongolia), African tick bite fever (Rickettsia africae: sub-Saharan Africa, West Indies, many Caribbean islands; Rickettsia aeschlimannii: Mediterranean region of Europe, Africa), European tick-borne lymphadenopathy (Rickettsia slovaca: Hungary, France; Rickettsia helvetica: Switzerland, Netherlands, France, Slovenia, Sweden; Rickettsia mongolotimonae: Europe), and Flinders Island spotted fever and Indian and Thai tick typhus (Rickettsia honei: southeastern Victoria, Flinders Island and Tasmania, Australia; India; Thailand; southern Texas, United States). Astrakhan fever Rickettsia is closely related to Israel tick typhus Rickettsia, and both are genotypes in the Rickettsia conorii complex. R. parkeri, which was previously thought to be nonpathogenic, is carried by Amblyomma maculatum ticks, and was identified as a cause of SFG rickettsial infection of people in the southern United States.159,161 A provisional agent, Rickettsia amblyommii, which is transmitted by Amblyomma americanum, is thought to cause a rickettsiosis similar to RMSF in North Carolina.7 cSerologic evidence of exposure exists; evidence of clinical illness associated with infection has been established by PCR in one instance.238 Rickettsia are vector-borne, obligate intracellular bacteria. These bacteria are important causes of emerging infectious disease in humans worldwide.164 Ticks serve as vectors for SFG Rickettsia with the exception of Rickettsia akari and Rickettsia felis. Vectors for these organisms are mites and fleas, respectively. There are more than 20 members of the SPG Rickettsia; most species of SFG Rickettsia have been discovered since the advent of genetic detection methods. These organisms are difficult to culture and study in the laboratory because of their obligate intracellular and endotheliotropic nature. Therefore, unlike most bacteria, it is difficult to differentiate species by phenotype in a clinical setting. Extensive serologic cross-reactivity between SFG Rickettsia further hinders easy differentiation among infecting species. Historically, the species of infecting SFG Rickettsia has been assumed based on geographic locale. Most spotted fever rickettsioses were presumed to be RMSF caused by R. rickettsii in the Western Hemisphere, and Mediterranean spotted fever (MSF), caused by Rickettsia conorii in Europe. Advances in molecular diagnostic techniques have led to the recognition and characterization of many novel species and Rickettsia previously considered to be nonpathogenic as disease-causing agents in people. In this chapter RMSF is emphasized as the model disease for SFG rickettsioses in dogs. Although not yet well characterized, it is likely that other SFG Rickettsia also cause disease in dogs, and that dogs may play a role as sentinels for their human companions, as they do for RMSF and MSF. The natural history and distribution of RMSF in the United States historically has centered primarily on the distribution of two ticks, Dermacentor andersoni and Dermacentor variabilis, which serve as natural hosts, reservoirs, and vectors for R. rickettsii. D. andersoni (the wood tick) is a three-host tick that is found in the region from the Cascade Mountains to the Rocky Mountains (Fig. 27-3). It is the principal vector of RMSF in the western United States and is also present in the Canadian provinces of southern British Columbia, Alberta, and Saskatchewan. D. variabilis (the American dog tick) is a three-host tick found from the Great Plains region eastward to the Atlantic coast of the United States and southern Canada. It has also been reported in California, southwestern Oregon, southern Washington, Idaho, and northern Mexico. Novel vectors have played a role in expanding the geographic distribution of RMSF in the United States. Rhipicephalus sanguineus (the brown dog tick) caused an outbreak of RMSF in a nonendemic area of Arizona.46,48a Infection existed in the dog population before the fatal outbreak in people.147,148 R. sanguineus is found throughout the United States, southern Canada, Mexico, and South America. Unlike the other vector ticks, R. sanguineus feeds on dogs during all three life stages. Although it prefers to feed on dogs, this tick inhabits buildings and will feed on people opportunistically. Increases in global temperature have been implicated in recent increases of feeding on humans.41,165 Other tick vectors also have the potential to transmit R. rickettsii in the United States. DNA of R. rickettsii has been found in Dermacentor occidentalis ticks in California. Although this tick has not yet been incriminated in the transmission of the disease, it does feed on humans.233 Because of the role of novel vectors in expanding the geographic range of R. rickettsii, veterinarians practicing in nonendemic areas of the United States should still be vigilant for the disease. Elsewhere in the Western Hemisphere, R. sanguineus and Amblyomma cajennense are the most commonly implicated ticks in transmission of R. rickettsii to people in Mexico or Central and South America, respectively. Dogs in Brazil have serum antibodies reactive to R. rickettsii and are sentinels for the disease (known as Brazilian spotted fever) in people.113,169 Experimental infection of dogs with the Brazilian strain has been reported, and clinical and laboratory findings are similar to those caused by R. rickettsii from North America.170 Clinical disease associated with infection in Brazilian dogs was reported for the first time.114 Although dogs in Brazil are strongly seropositive for antibodies to R. rickettsii169,189 and other SFG rickettsiae,113 the former organism has not been detected by polymerase chain reaction (PCR) in a very limited sampling of A. cajennense ticks from regions where high rates of positive serologic test results in dogs and horses have been found.189 In other areas of Brazil, the organism was identified from Amblyomma aureolatum, which may serve as a vector for some regions of infection. Ixodid ticks feed once during each life stage, and feeding on successive hosts successfully transmits the infection to new mammalian hosts. Ticks become infected by two means (Fig. 27-4). First, horizontal transmission can occur as noninfected ticks feed on certain small mammals, including chipmunks, voles, and ground squirrels, that develop sufficient rickettsemia during acute infection. The primary sylvan cycle, which maintains the transmission cycle in nature, occurs among these small rodents and immature (larval and nymphal) tick stages, and it is possible that medium-size mammals such as raccoons, opossums, and foxes are additional sources for infecting ticks. Although of minor importance, birds are a means by which infected ticks can be transported into new areas. Second, ticks can be infected transstadially and also by transovarial passage between generations. Venereal transmission of R. rickettsii can also occur when adult ticks copulate201; however, female ticks that are infected in this manner do not transmit infection transovarially. R. rickettsii initially replicates in the epithelial cells of the tick midgut, enters the hemocoel, and from there spreads to and multiplies in other tick tissues, including the salivary glands and ovaries. Ticks must ingest numerous rickettsiae for successful transovarial transmission, which is the primary means by which R. rickettsii is maintained in nature. R. rickettsii usually enter the body through the bites of infected ticks (Fig. 27-5). Rickettsiae are disseminated via the circulatory system and invade and replicate in endothelial cells of smaller arteries and venules. Phospholipase and proteases have been incriminated as mechanisms for rickettsial damage to cell membranes. Subsequent endothelial cell damage initiates a vasculitis with platelet activation and activation of the coagulation system accompanied by decreased plasma levels of antithrombin III and plasminogen and increased fibrinogen degradation products. These hematologic changes are consistent with simultaneous activation of the fibrinolytic and coagulation systems. Coagulation factor consumption is not usually extensive enough to cause hypofibrinogenemia or overt disseminated intravascular coagulation (DIC).225 Results of coagulation factor analysis suggest activation of the extrinsic and intrinsic pathways. Elevated platelet-associated immunoglobulin levels in dogs with experimentally and naturally induced RMSF suggest an immune-mediated cause of the thrombocytopenia.76 Progressive necrotizing vasculitis may be caused sequentially by complement activation, cellular chemotaxis, and subsequent vascular necrosis, and extravasation of blood and coagulatory consumption can contribute to thrombocytopenia. Organs with endarterial circulation, such as the skin, brain, heart, and kidneys, are most adversely affected by thrombosis. Clinical and subclinical illnesses have been reported in dogs with naturally occurring and experimentally produced RMSF. Naturally acquired immunity has an important role in protection against clinical illness, and R. rickettsii appears to be cleared from the body after recovery from natural infection. Anti-SFG rickettsial antibodies can be found in the healthy canine population in endemic areas. This observation may be a consequence of subclinical infections or the result of exposure to nonpathogenic SFG rickettsiae in ticks. Immunogenic contact with R. rickettsii induces a protective response in experimental dogs to reinfection for at least up to 3 years.26 Most dogs are examined for illness during the months of March to October. Purebred dogs appear to be more prone to develop clinical illness than mixed-breed dogs, and the German shepherd dog has a particularly high prevalence of disease. People with glucose-6-phosphate dehydrogenase deficiency and English springer spaniels with suspected phosphofructokinase deficiency have a more fulminant course of illness and are more likely to develop dermal necrosis,231 as are animals in which treatment is delayed. Clinical signs of illness in dogs are similar to those of naturally infected people (Box 27-1 and Web Table 27-1). WEB TABLE 27-1 Frequency of Clinical Findings in People and Dogs with RMSF CNS, Central nervous system; NR, not reported or not stated as such; RMSF, Rocky Mountain spotted fever. dTemperature >39.5° C (103° F). eInclude vestibular signs of nystagmus, head tilt, circling, and incoordination. fIncludes ocular discharge, scleral and conjunctival congestion, scleral and conjunctival hemorrhage, conjunctivitis, scleral petechiae, anterior uveitis, hyphema, iridal hemorrhage, and retinitis. Fever, one of the first and most consistent findings, can occur within 2 to 3 days after tick attachment. The range of the incubation period is 2 to 14 days. Early cutaneous lesions in some dogs consist of edema and hyperemia of the lips, penile sheath, scrotum, pinna, other extremities (Fig. 27-6), and rarely the ventral abdomen. Discrete clear vesicles and focal erythematous macules have been observed on the buccal mucosa. Male dogs that develop scrotal edema or epididymal swelling often have a stiff gait and are reluctant to walk. Gait abnormalities are a result of inflammation of joints, muscles, and meninges. Petechial and ecchymotic hemorrhages may develop subsequent to the acute illness in some dogs and, if present, occur on ocular, oral, and genital mucous membranes rather than the skin (Fig. 27-7). Funduscopic examination provides a more sensitive means of detecting these hemorrhagic lesions (see Fig. 92-22).45 Epistaxis, melena, and hematuria may be noted in severely affected animals. Neurologic signs of generalized cerebral and spinal cord involvement have been found (see Box 27-1). More severe neurologic abnormalities caused by meningitis progressing to encephalomyelitis are found in dogs and people in whom the diagnosis and treatment are delayed. Focal or localizing neurologic signs, such as vestibular disease, are common in the early phases of illness and are often the reason the animal is taken to the veterinarian. Infected dogs may make a rapid and complete recovery if they are mildly affected or antimicrobial therapy is instituted early. Permanent organ damage, particularly resulting in residual neurologic dysfunction, may occur within 1 to 2 weeks of onset of clinical signs in severely affected dogs that survive the acute stages of illness. Necrosis of the extremities and previously hyperemic or edematous portions of the body may occur at this time (Fig. 27-8). Dogs may die in the acute stages of illness as a result of hemorrhagic diathesis or from thrombosis of vital organs. Cardiovascular, neurologic, and renal damage are the most consistent causes of death or permanent organ dysfunction. Death in some severely affected dogs has been caused by disseminated, rapidly progressing meningoencephalitis. Shock, cardiovascular collapse, and oliguria become apparent in the terminal stages of illness. Thrombocytopenia is one of the most consistent hematologic abnormalities in infected dogs. Platelet counts generally range from 23,000/µL to 220,000/µL. Megathrombocytosis is usually detectable in a majority of cases. Thrombocytopenia in RMSF is often not less than 75,000/µL; however, lower trends have been reported.67 Electronic counting of thrombocytopenic specimens below this level generally gives lower counts than hemocytometer methods on the same specimen when correlations are made. A mild prolongation of the activated clotting time may be the only coagulation abnormality.43,44 Rarely, overt DIC characterized by prolongation of the activated coagulation time, prothrombin time, activated partial thromboplastin time, and thrombin time with elevated fibrinogen degradation products occurs in dogs.45 Biochemical abnormalities may include mildly increased serum glucose concentration and elevated serum alkaline phosphatase, alanine transferase, and aspartate transferase activities. Hypercholesterolemia has been one of the more consistent findings in affected dogs. Hypoalbuminemia is often observed and probably is caused by leakage associated with generalized vascular endothelial damage. Hyponatremia, hypochloremia, and metabolic acidosis are variable findings. The serum urea nitrogen may increase in terminal stages of the disease corresponding with oliguria and renal failure. Proteinuria and hematuria occur as a result of incoagulability or glomerular and tubular injury. Proteinuria in the absence of leukocytes or erythrocytes in the urine indicates tubular or glomerular sources. Bilirubinuria and hyperbilirubinemia occur in some dogs, but these are usually mild. Serum creatine kinase has been mildly to moderately increased in some dogs. Cerebrospinal fluid analysis results are frequently within reference limits; however, mildly increased protein (25 to 150 mg/dL; reference less than 25 mg/dL) and polymorphonuclear and mononuclear cells (5 to 50 total nucleated cells/µL; reference less than 5 total nucleated cells/µL) may be found. Analysis of synovial fluid in dogs with accompanying polyarthritis has shown inflammatory changes, with a predominant increase in neutrophils. Results of lupus erythematosus cell, rheumatoid factor, antinuclear antibody, platelet autoantibody, and Coombs’ testing and of bacterial blood culture are usually negative. The presence of co-infections with other tick-borne agents such as Ehrlichia or Babesia organisms may cause positive Coombs’ test results (see Chapters 26 and 76, respectively). If present, electrocardiographic abnormalities consist of sinoatrial node dysfunction, ST-segment and T-wave depressions, and premature ventricular contractions. Thoracic radiography reveals diffuse increased interstitial density, especially in dogs having dyspnea and coughing.51 Although CNS signs are typical of SFG rickettsial infections, fibrillation potentials on electromyography have been reported in one dog with concurrent CNS manifestations of RMSF142 and pronounced lower motor neuron paralysis has been observed in MSF infection in people.213 Whether this electrical evidence of distal axonal denervation results from polymyositis and/or polyneuritis remains to be established; however, the signs resolve after antirickettsial therapy. Measurement of R. rickettsii serum antibodies is the primary method of confirmation of RMSF in clinical practice. Mammalian hosts differ in regard to the specificity of their immune response to rickettsiae. Well-adapted hosts, such as mice, develop highly specific antibody titers against each species of organism. Human serologic responses generally react strongly with group-reactive antibodies but do not consistently distinguish between Typhus Group and SFG rickettsiae. Seroreactivity of dogs appears to be between that of rodents and people. Cross-reactions in dog sera develop between SFG antigens; however, the titer is generally highest to the specific rickettsial species causing infection. Generally, minimal reactions to Typhus Group or other rickettsiae occur. Clinically healthy dogs on the Atlantic seaboard have between 5% and 15% seropositivity to SFG rickettsiae, indicating infection by antigenically related avirulent rickettsiae or subclinical infection. Some ill dogs have shown stronger reactivity to nonpathogenic Rickettsia bellii and Typhus Group antigens than to R. rickettsii, suggesting that another yet unidentified Rickettsia organism may be pathogenic.24 Several serologic tests have been developed to detect antibodies to RMSF in people, including Weil-Felix test, complement fixation, microscopic immunofluorescence (Micro-IF), microagglutination, indirect hemagglutination, enzyme-linked immunosorbent assay (ELISA), and latex agglutination.95 The Micro-IF, ELISA, and latex agglutination tests appear to be the best suited to examine canine sera.74 An increased IgM titer or fourfold increase in IgG titer is needed to confirm active infection with R. rickettsii. The Micro-IF test, an indirect fluorescent antibody (FA) test, and the ELISA have the advantages of requiring small amounts of sera and reagents, having high sensitivity, and classifying antibodies to R. rickettsii as IgM or IgG. Measurement of IgG antibodies to R. rickettsii using the Micro-IF method is used by most diagnostic laboratories that test canine sera (see Web Appendix 5). Acute and convalescent IgG titers have been generally less than 64 in uninfected dogs and in those with ehrlichiosis, although titers vary among laboratories. Actively infected dogs may or may not have increased titers (≥64) by the time clinical signs are apparent and the first sample is taken. IgG titers in experimentally infected dogs with disease manifestations by 3 to 6 days after infection have not increased until 2 to 3 weeks after infection. Therefore, seronegative results do not discount the possibility of RMSF, and a convalescent sample should be tested at a later date. Dogs with neurologic dysfunction are more likely to have increased serologic titers at the time of examination, because these signs are typically in more advanced stages of illness. An IgG titer increase of fourfold or greater is required to document active infection definitively. High IgG titers that develop in actively infected dogs generally decrease after 3 to 5 months, although some may remain elevated (≥128) for at least 10 months.60,75 Thus, unless single titers are markedly increased (≥1024), active infection cannot be absolutely ascertained, and paired samples must be obtained. Differences can be found among laboratories performing the test and within laboratories testing the same sample at a later date. Therefore it is recommended that acute and convalescent serum samples be submitted together. Simultaneous assessment of IgM and IgG titers can provide more accurate assessment as to the time course of infection when evaluating a single serum sample. Seroconversion to R. rickettsii antigen is suppressed by administration of antimicrobials early in the disease.27 The latex agglutination test appears to be a rapid and specific assay for recent R. rickettsii infection in dogs.75 Because the sensitivity is lower than the Micro-IF test, false-negative results occur, although a single increased latex agglutination titer (32) appears to be diagnostic for RMSF in dogs. The advantage of the direct FA procedure on tissue is that it may be able to confirm the diagnosis on a single sample as early as the third or fourth day of disease. Direct FA procedures can be performed by veterinary or human laboratories without regard to host species differences. Few false-positive results are found with the direct FA test, but many (30% to 40%) false-negative reactions can occur and are usually a result of prior therapy with chloramphenicol or tetracycline or failure to obtain a sample through an area of vasculitis. Specimens should be obtained early in the course of infection because organisms are eliminated from the tissue within a few days, especially after institution of antimicrobial therapy. Because of the usual absence of cutaneous petechiae in dogs, biopsy specimens could be obtained from mucosal hemorrhages or from vesicles that may develop in the buccal mucosa. Adaptation of this method using immunoperoxidase staining increases the test sensitivity and specificity.120 Immunohistochemical methods have been used to substantiate infection in people dying of RMSF that was not detected by serologic means.160 PCR has become the reference method for confirming infection in patient blood samples. Many genes have been targeted and different PCR methodologies have been used to detect SFG Rickettsia infection in people.34,58,64,122 Some assays can detect as little as one copy of target DNA per reaction in vitro. However, sensitivities range from 14% to 71% when patient serum samples are tested, presumably because of low numbers of circulating organisms in peripheral blood.34,122 A nested PCR that amplifies a portion of the 16srDNA gene is more sensitive than culture in detecting rickettsial DNA in dogs after treatment and remains positive for longer than culture, possibly because of the persistence of nonviable nucleic acid.27 Another PCR developed to detect SFG Rickettsia in dog blood amplifies a region of the SFG Rickettsia ompA gene.108 Sensitivity of the conventional PCR is 100% for 15 copies and 45% for 3 copies of SFG Rickettsia DNA in vitro, when spiked samples of DNA extracted from healthy dog blood are tested. The sensitivity of the quantitative adaptation of this PCR is 96% for 5 copies of target DNA. Using the conventional PCR, Rickettsia DNA was detected in 5 of 6 (83%) tests of blood samples from dogs with negative serum antibody titer results, and 2 of 9 (22%) dogs with positive serum antibody titer results. Thus, as is the case in people, PCR can confirm infection, but a negative test result does not eliminate the disease from consideration. PCR is also used to detect the organisms in tissue specimens, ticks, and for comparison of isolates.200,222 Some PCRs can be used to differentiate among SFG Rickettsia species infecting people and dogs.61,108,108 PCR has also documented that many dogs infected with R. rickettsii may be coinfected with various Ehrlichia, Bartonella, Babesia, other Rickettsia species, or a combination of these, making a specific diagnosis uncertain and causing higher morbidity than with a single-agent infection.111
Rocky Mountain and Mediterranean Spotted Fevers, Cat-Flea Typhuslike Illness, Rickettsialpox, and Typhus
Rocky Mountain Spotted Fever
Etiology and Epidemiology
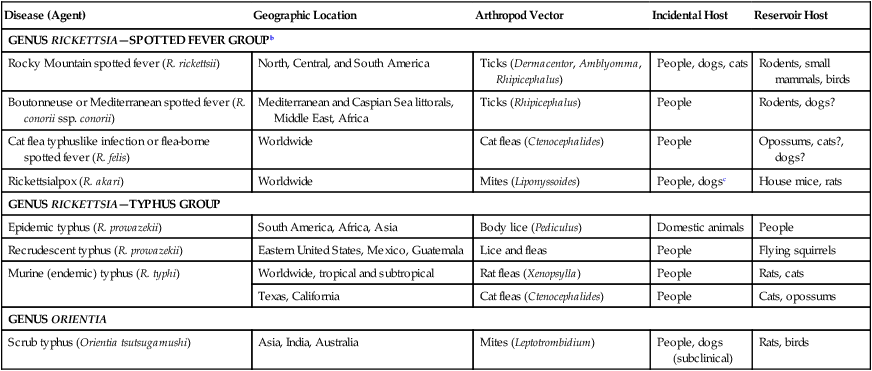
Disease (Agent)
Geographic Location
Arthropod Vector
Incidental Host
Reservoir Host
GENUS RICKETTSIA—SPOTTED FEVER GROUPb
Rocky Mountain spotted fever (R. rickettsii)
North, Central, and South America
Ticks (Dermacentor, Amblyomma, Rhipicephalus)
People, dogs, cats
Rodents, small mammals, birds
Boutonneuse or Mediterranean spotted fever (R. conorii ssp. conorii)
Mediterranean and Caspian Sea littorals, Middle East, Africa
Ticks (Rhipicephalus)
People
Rodents, dogs?
Cat flea typhuslike infection or flea-borne spotted fever (R. felis)
Worldwide
Cat fleas (Ctenocephalides)
People
Opossums, cats?, dogs?
Rickettsialpox (R. akari)
Worldwide
Mites (Liponyssoides)
People, dogsc
House mice, rats
GENUS RICKETTSIA—TYPHUS GROUP
Epidemic typhus (R. prowazekii)
South America, Africa, Asia
Body lice (Pediculus)
Domestic animals
People
Recrudescent typhus (R. prowazekii)
Eastern United States, Mexico, Guatemala
Lice and fleas
People
Flying squirrels
Murine (endemic) typhus (R. typhi)
Worldwide, tropical and subtropical
Rat fleas (Xenopsylla)
People
Rats, cats
Texas, California
Cat fleas (Ctenocephalides)
People
Cats, opossums
GENUS ORIENTIA
Scrub typhus (Orientia tsutsugamushi)
Asia, India, Australia
Mites (Leptotrombidium)
People, dogs (subclinical)
Rats, birds
Pathogenesis
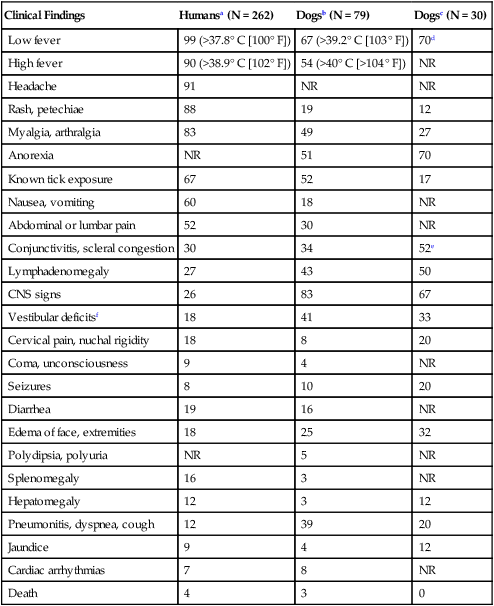
Clinical Findings
Clinical Findings
Humansa (N = 262)
Dogsb (N = 79)
Dogsc (N = 30)
Low fever
99 (>37.8° C [100° F])
67 (>39.2° C [103° F])
70d
High fever
90 (>38.9° C [102° F])
54 (>40° C [>104° F])
NR
Headache
91
NR
NR
Rash, petechiae
88
19
12
Myalgia, arthralgia
83
49
27
Anorexia
NR
51
70
Known tick exposure
67
52
17
Nausea, vomiting
60
18
NR
Abdominal or lumbar pain
52
30
NR
Conjunctivitis, scleral congestion
30
34
52e
Lymphadenomegaly
27
43
50
CNS signs
26
83
67
Vestibular deficitsf
18
41
33
Cervical pain, nuchal rigidity
18
8
20
Coma, unconsciousness
9
4
NR
Seizures
8
10
20
Diarrhea
19
16
NR
Edema of face, extremities
18
25
32
Polydipsia, polyuria
NR
5
NR
Splenomegaly
16
3
NR
Hepatomegaly
12
3
12
Pneumonitis, dyspnea, cough
12
39
20
Jaundice
9
4
12
Cardiac arrhythmias
7
8
NR
Death
4
3
0
Diagnosis
Clinical Laboratory Findings
Serologic Testing
Direct Immunodetection
Genetic Detection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Rocky Mountain and Mediterranean Spotted Fevers, Cat-Flea Typhuslike Illness, Rickettsialpox, and Typhus