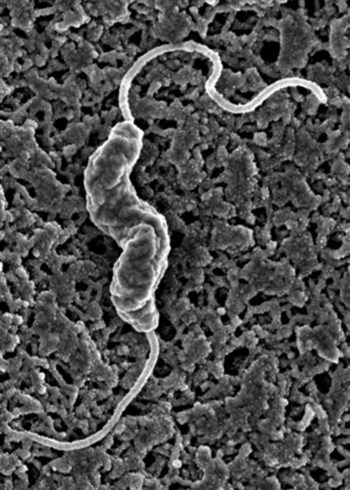
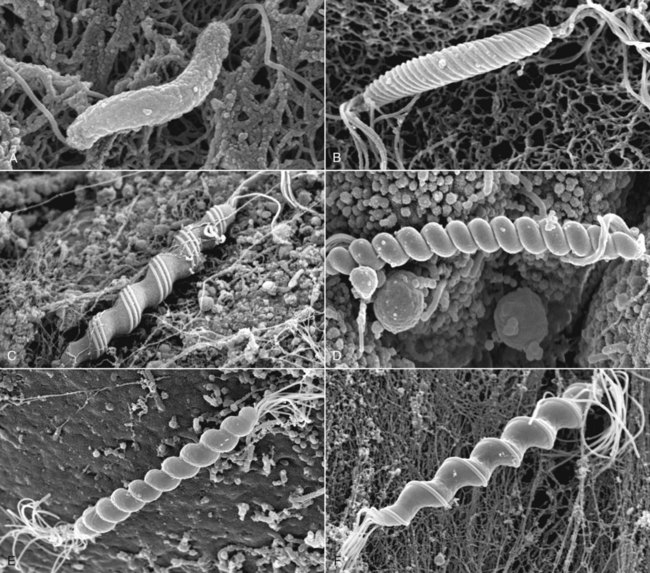
Campylobacter is a genus of gram-negative, slender, curved, motile rods (1.5 to 5 µm × 0.2 to 0.5 µm) that are found singularly, in pairs, or in chains with three to five spirals (Fig. 37-1). The cells can also be curved, S = shaped, or gull-shaped. Campylobacter species have a single, nonsheathed polar flagellum and microaerobic growth requirements. Campylobacter jejuni is the organism routinely associated with diarrheal disease in dogs, cats, and humans, as well as other domestic, wild, and laboratory animals. Campylobacter coli, distinguished from C. jejuni on the basis of hippurate hydrolysis, is also isolated from diarrheic animals and humans. Other intestinal catalase-negative campylobacters, Campylobacter upsaliensis, Campylobacter helveticus, and Campylobacter lari, have been increasingly isolated from asymptomatic and diarrheic dogs and cats.* Results of further genetic analysis indicate that dogs may also be colonized with C. felis and Campylobacter showae in addition to other Campylobacter spp. previously identified by culture.77 In addition to the isolation of multiple species of Campylobacter, genetic heterogeneity may also exist in individual Campylobacter species isolates from the feces of individual animals.3,324,325,330a Web Table 37-1 summarizes the features of these Campylobacter species. WEB TABLE 37-1 Comparative Features of Campylobacter Species that Infect Dogs or Cats Privately owned adult dogs and cats generally have a lower isolation rate of C. jejuni than strays or those maintained in kennels or catteries, laboratories, and animal shelters.4,193 C. jejuni has been isolated from 21% and 29% of diarrheic cats and dogs, respectively, compared with 4% of clinically healthy cats and dogs.114 In other studies the isolation rate from feces of mature dogs and cats with and without diarrhea has varied from 0% to 50%.44,569 Prevalence of C. jejuni in clinically healthy dogs in one report was significantly greater (p less than 0.05) in dogs younger than 6 months old, in dogs living in high-density and cohabitation housing conditions for long periods, and in the autumn months.604 In a longitudinal study conducted in Denmark on 26 young (3 months to 1 year of age) pet dogs, C. jejuni was more prevalent in the feces of these dogs compared to those between 1 and 2 years of age.245 The authors also documented that dogs were often colonized with C. upsaliensis for up to 21 months of age. In Cheshire, England, high risk factors for carriage of C. upsaliensis by a dog included living with another dog that also carried C. upsaliensis; being small rather than medium-sized; being less than 3 years of age; living in a household that kept fish; being fed commercial dog treats; and being fed human food tidbits, particularly in the dog’s bowl.653 A higher prevalence of C. jejuni and other enteric bacteria has been found in dogs being fed raw meat.364 In clinically healthy or diseased dogs being evaluated at veterinary practices in the United Kingdom, C. upsaliensis isolation rate overshadowed that of C. jejuni, and most of the isolates were made from younger dogs.454 In a 6-year study, 64 of 227 commercially reared cats without clinical signs of diarrhea had microaerobic bacteria isolated from their feces.533 All the isolates were initially identified as Campylobacter-like organisms (CLOs) based on biochemical and phenotypic characteristics. DNA extractions from 51 of these isolates were subjected to the polymerase chain reaction (PCR) using primers specific for Helicobacter spp. and Campylobacter spp. Of these isolates, 92% (47 isolates) were positive for Campylobacter spp., 41% (21 isolates) were positive for Helicobacter spp., 33% (17 isolates) were positive for both genera, 59% (30 isolates) were positive only for Campylobacter spp., and 8%8 were positive only for Helicobacter spp. Sixteen of the 47 Campylobacter-positive cultures were positive for more than one species of Campylobacter. Based on a species-specific PCR assay, 83% of the isolates were identified as C. helveticus, 47% of the isolates were identified as C. upsaliensis, and 6% of the isolates were classified as C. jejuni. This study demonstrated that biochemical and phenotypic characteristics of microaerobic organisms in cat feces were insufficient to characterize mixed Helicobacter and Campylobacter infections. The finding of C. helveticus in a high percentage of the cats confirms the findings of a previous study in England in which the organism was cultured from the feces of healthy cats.565 In another investigation on co-infection with Campylobacter spp., enteric Helicobacter spp. and Anaerobiospirillum spp. in healthy or diarrheic dogs and cats, 27 animals (10%) were found to be infected with Campylobacter spp. and Helicobacter spp., and 8 dogs and 2 cats harbored all three genera.500 C. upsaliensis was the most frequently isolated species in dogs and C. helveticus from cats, whereas the isolation rates of C. jejuni were similar in both animals. The most frequent isolates were Helicobacter bilis (15.3%) followed by Helicobacter canis (12%) and finally Helicobacter cinaedi (6.2%). No statistically significant correlation between isolation of single or mixed infections and the presence of diarrhea was observed. In one report, 361 specimens were collected by rectal swab from cats and dogs in Ireland (179 cats and dogs in shelters and 182 cats and dogs in a university veterinary hospital); 163 of 361 (45.2%) of the pets surveyed were positive for Campylobacter spp. using a combination of culture techniques.1 Specification results using DNA extraction and specific PCR confirmed that 50% of the isolates for C. upsaliensis and 41.9% were C. jejuni. A smaller percentage of isolates were C. coli (2.6%), C. lari (1.5%), and C. helveticus (1.1%). For 2.9%, identification among C. jejuni, C. lari, and C. coli could not be made.1 In a study conducted in pet dogs and cats in Barbados, C. jejuni was cultured from 34 of 66 dogs (51.5%), whereas C. upsaliensis was isolated in 6 of 20 (30%) and C. helveticus in 10 of 20 (50%) of cats, respectively.665 As with most enteric microbial pathogens, fecal-oral spread with foodborne and waterborne transmission appears to be the principal avenue for infection. Suspected sources of Campylobacter infection include contaminated meat products, particularly poultry and unpasteurized milk.407 Asymptomatic carriers can shed campylobacters in their feces for prolonged periods and directly infect other animals or contaminate food products or water. Nosocomial infection of hospitalized animals occurs, as does exposure through other pets in a household (e.g., ferrets, hamsters, birds, rabbits) and rural farm animals that may shed the organism. Flies have also been incriminated in the spread of organisms.428 The severity of the disease depends on the number of organisms ingested by the host as well as previous exposure and development of protective antibody. Other enteric pathogens, such as parvovirus and coronavirus, Giardia organisms, or Salmonella organisms, may play a synergistic role.64,511 Environmental, physiologic, and surgical stresses may also exacerbate the severity of the disease. Blood and leukocytes in the feces, congestion, edema, mucosal ulcers, and occasional sepsis in humans suggest that the organism can be invasive. Experimental challenge in laboratory animals also indicates that the organism can be isolated from blood several days after challenge inoculation. Experimental infections of puppies and kittens with strains isolated from humans with diarrhea are less severe than those observed in the humans from which the organisms were isolated. Animals appear to be more resistant or better adapted to the pathogenic effects of various different C. jejuni isolates and usually only develop watery mucoid diarrhea.372,478,478 A variety of virulence factors, such as enterotoxins, cytotoxins, or adherence or invasion properties, are expressed by different C. jejuni–coli isolates. A cytotoxin, referred to as cytolethal distending toxin (cdt), has been identified in C. jejuni; however, its role in production of intestinal disease is in the process of being determined. For example, wild-type C. jejuni containing cdt cause gastroenteritis in infected nuclear factor kappa B (NF-κB) deficient mice, whereas isogenic C. jejuni mutants lacking cdt do not induce disease.196 In vitro, the cytotoxin causes distention of cell lines and cell-cycle arrest in the G2M1 phase in the cell cycle. An intriguing report in humans links the occurrence of acute onset of Campylobacter– or Salmonella-induced diarrhea to subsequent development of inflammatory bowel disease (IBD).225 Whether such an association occurs in dogs and cats is presently unknown. In many cases, dogs are asymptomatic carriers of Campylobacter species. The clinical syndrome occurs most frequently in dogs younger than 6 months. Animals may be more susceptible to clinical disease when stressed by hospitalization, concurrent disease, pregnancy, shipment, or surgery. Campylobacter-associated diarrhea has a wide clinical spectrum in dogs as well as humans, ranging from mild, loose feces to watery diarrhea to bloody mucoid diarrhea. Acute campylobacteriosis that develops in puppies and some adult dogs is manifest by mucus-laden, watery, or bile-streaked diarrhea (with or without blood and leukocytes) for 5 to 15 days, partial anorexia, and occasional vomiting.187,189,189 Elevated temperature and leukocytosis may also be present. In certain cases, diarrhea can be chronic and last 2 or more weeks, can be intermittent, or in some cases can be present for several months.158 In humans, C. jejuni can cause extraintestinal complications such as arthritis, meningitis, myocarditis, cholecystitis, and abortions. C. jejuni has been isolated from two dogs with bacteremia and cholecystitis.443 Clinical signs included anorexia, fever, and icterus. Ultrasonography showed a fluid-filled, abnormally thickened gallbladder wall in both dogs. C. jejuni and Campylobacter fetus are also recovered, although infrequently, from the bile of humans with cholecystitis.216,628 Insofar as Helicobacter species are present in liver and bile of various hosts (see Intestinal and Hepatic Helicobacters section in this chapter), detailed biochemical and phenotypic descriptions are necessary to fully characterize and validate whether microaerophilic organisms isolated from the hepatobiliary tract of dogs are Campylobacter or Helicobacter species. Campylobacter-associated abortion has also been noted in dogs, although it is infrequent. In cats, clinical signs of campylobacteriosis are poorly documented in the absence of other pathogens. As with dogs, campylobacteriosis infected cats are usually clinically healthy. If clinical signs are evident, the animal generally is younger than 6 months. In a prevalence survey of 159 cats from pounds, 17 shed C. jejuni in the feces, but of these only 2 had bloody, mucus-laden diarrhea. Giardia species were present in both, combined with Isospora species in one cat and Toxocara species in the other. Another cat concurrently infected with Salmonella species and C. jejuni was depressed and anorectic but not diarrheic. Culture results from the two cats’ feces after antibacterial therapy and clinical improvement were negative for C. jejuni.173 Chronic diarrhea in another cat that had serum antibodies to C. jejuni plus positive culture results for the organism abated when treated with chloramphenicol. C. jejuni could not be recultured from the stool after therapy.176 Rapid presumptive diagnosis is possible using either dark-field or phase-contrast microscopy. Fresh fecal samples are examined for curved bacteria with the characteristic darting motility of C. jejuni. This method is especially sensitive in humans (and perhaps dogs and cats) during the acute stage of clinical diarrhea. With Gram stain, faintly staining, gram-negative, gullwing–shaped slender rods are apparent. Maintaining the safranin counterstaining improves their visualization.394 However, based on morphology alone, these organisms could also be enteric helicobacters. Presence of fecal leukocytes should be ascertained, because leukocytes may be found in enteritis caused by natural or experimental infections with C. jejuni. Rectal swab specimens can be obtained, or fresh feces can be collected. For diagnosis of C. jejuni cholecystitis and bacteremia, appropriate diagnostic testing, including abdominal ultrasonography, gallbladder aspiration with bile culture, and blood culture, should be performed.443 Transport of fecal specimens usually does not present isolation difficulties because C. jejuni-coli remain viable in feces at room temperature for at least 3 days and at refrigeration temperatures for at least 1 week. However, higher rates of isolation can be achieved with shorter time delays. In a survey of pets in Ireland using five different selective culture techniques, modified charcoal cefoperazone deoxycholate (MCCDA) basal agar with cefoperazone amphotericin and teicoplanin (CAT) selective supplement (Oxoid) proved to be the method of choice for isolation of the most common campylobacters detected in pets and humans.2 A diagnostic biochemical test strip (API Campy strips, bioMerieux Industry, Marcy l’Étoile, France) for speciation of campylobacters is now commercially available (see Web Appendix 6). Plates are incubated in a microaerobic atmosphere at 37° C and 42° C and examined at 72 to 96 hours. Colonies composed of curved gram-negative rods are round, raised, translucent, and sometimes mucoid. Isolates are identified as C. jejuni on the basis of positive oxidase and catalase reactions, susceptibility to nalidixic acid, resistance to cephalothin, and inability to grow at 42° C under aerobic conditions. C. upsaliensis and C. helveticus are catalase negative, and isolation is enhanced by selective filtration of feces to be cultured through a 0.45-µm filter.223 Investigators in South Africa have published results using a protocol that has been in use in their diagnostic laboratory since 1990, which allows primary isolation of multiple species of Campylobacter and Helicobacter from the diarrheic specimens of individual children.340,341 Filtrates are plated onto antibacterial-free blood agar plates and incubated in an H2-enriched atmosphere. These authors not only documented an increase in the number of isolated CLOs but also were able to culture C. upsaliensis for the first time. They have reported a 16.2% prevalence of multiple species of CLOs based on primary isolation, biochemical characterization, and serologic confirmation. They frequently recovered between two and five species of CLOs from one stool sample, with C. jejuni (different serotypes), C. coli, C. upsaliensis, Helicobacter fennelliae, and H. cinaedi being commonly isolated.340 Additional analysis using the filtration isolation technique with cat and dog feces may yield prevalence rates for mixed Helicobacter and Campylobacter infections even higher than those reported in dogs500 and cats.533 Various procedures have been used, especially during outbreaks, to identify different serotypes of C. jejuni-coli by using thermostable and thermolabile surface antigens.400 Isolates from humans and various animal species have shown that extensive serologic heterogeneity exists within C. jejuni-coli. Many of the isolates frequently found in diarrheic and normal dogs and cats have had serotypes frequently encountered in human patients.177 For example, serotype 4 (commonly associated with outbreaks of C. jejuni–associated diarrhea in humans) was also a common serotype isolated from commercially reared beagles.177,590 A variety of techniques can detect serum antibodies to various antigens of Campylobacter. A specific bactericidal assay has been used to demonstrate a rising antibody titer in humans and animals. Other serologic assays, such as the enzyme-linked immunosorbent assay (ELISA), have been developed to survey human populations during outbreaks of campylobacteriosis and ascertain previous exposures to the organism. Unfortunately, no systematic studies have been performed in dogs and cats to ascertain the importance of antibody titers as an indicator of infection in animals with or without diarrhea. Plasmid characterization, restriction enzyme analysis, and ribotyping can also be used for strain identification, but these techniques require specialized methodologies. The technique of restriction enzyme analysis of whole genomic DNA allowed verification of C. jejuni zoonotic transmission to personnel in vivaria caring for wild-caught and captive coyotes.199 A multiplex PCR assay for identification of and differentiation of C. jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus ssp. fetus also has been developed.634 Importantly, primers specific for 23SrRNA can be used as internal controls to identify the closely related genera Campylobacter, Arcobacter, and Helicobacter.634 Rapid identification of campylobacters, arcobacters, and helicobacters can also be accomplished on clinical isolates using PCR-restriction fragment length polymorphism analysis of the 16SrRNA gene.388 Grossly visible findings in naturally and experimentally infected canine neonates are abnormally fluid colonic contents as well as thickening, congestion, and edema of the colonic mucosa.92 Microscopic abnormalities in the colon and cecum are decreases in epithelial cell height, brush borders, and numbers of goblet cells. Hyperplasia of epithelial glands results in a thickened mucosa. Subepithelial congestion, hemorrhage, and inflammatory infiltrates have also been seen. Findings in adult animals inoculated with C. jejuni were similar to those in some dogs with natural campylobacteriosis: stunting of intestinal villi, infiltration of lamina propria with inflammatory cells, and hyperplastic Peyer’s patches.372 Naturally occurring intestinal lesions in adult dogs have consisted of mucosal hyperplasia in the colon characterized by immature hyperchromatic, hyperplastic epithelial cells with a high mitotic index and deep and irregular crypts.187 With Warthin-Starry stain, CLOs have been demonstrated attached to but not within colonic epithelium. However, in experimentally produced C. jejuni colitis in macaques, intraepithelial invasion of C. jejuni was demonstrated by electron microscopy (EM).504 A relative increase had been noted in the number of lymphocytes infiltrating the lamina propria. Ileal lesions consisted of focal, shallow crypts and blunt, irregular villi, which occasionally fused. Mild congestion and dilatation of lacteals have been found. Stunting and fusing of intestinal villi and mononuclear cell infiltrates in lamina propria have also been noted in subacute stages of parvovirus infection and in a dog with protracted Campylobacter-associated diarrhea.102 Experimentally, selected strains of C. jejuni produce hepatitis in mice, and the organism has been isolated from inflamed livers of dogs. With experimental C. jejuni infection in rhesus monkeys, the liver and gallbladder were found to be the organs most consistently colonized, after the intestine.156 The efficacy of antibacterial therapy and treatment of Campylobacter-associated diarrhea in the dog and cat is not known, nor is it known whether antibacterials effectively alter the course of enteric disease. However, in some dogs and cats with severe diarrhea, antibacterial therapy may be warranted. Antibacterial treatment of infected animals may be instituted to minimize exposure to people and other pets in the household. Fortunately, strains of Campylobacter isolated from animals and people are susceptible to several antimicrobial agents (Table 37-1). Erythromycin, the drug of choice for Campylobacter-induced diarrhea in people, may also be effective in the treatment of the disease in animals. Treatment of clinically affected cats and dogs has resulted in resolution of the illness and elimination of the organism as determined by Campylobacter-negative fecal culture results. However, failure to eliminate C. jejuni with oral erythromycin from ferrets housed in a research environment has also been noted.172 Erythromycin can also cause gastric irritation and vomiting in some animals. TABLE 37-1 Drug Therapy for Nonenteric Salmonellosis and Other Enteric Bacterial Infections in Dogs and Cats B, Both dog and cat; C, cat; CDI, Clostridium difficile infection; CPAD, Clostridium perfringens–associated diarrhea; D, dog; IM, intramuscular; IV, intravascular; PO, by mouth; SC, subcutaneous. aSee the Drug Formulary in the Appendix for additional information on each drug. bDose per administration at specified interval. dOther quinolones such as ciprofloxacin, difloxacin, or marbofloxacin are alternatives, although dosages vary. Chloramphenicol has been given with mixed results to treat Campylobacter-associated diarrhea in dogs and cats. Treatment in dogs has resulted in abatement of clinical signs. However, the same organism has been reisolated after therapy has been completed. It is possible that these dogs developed an antibacterial-induced carrier state (as occurs in enteric Salmonella infections), experienced a protracted period of Campylobacter shedding, or became reinfected. Clinical improvement was noted in a diarrheic cat treated with chloramphenicol, and fecal culture results after completion of the treatment were negative for C. jejuni. C. jejuni–associated bacteremia and cholecystitis have been successfully treated with intravenous cefoxitin, a second-generation cephalosporin, or oral erythromycin (see Table 37-1) for 21 days, which resulted in complete resolution of all clinical and laboratory abnormalities.443 Several other antibacterials are active against Campylobacter strains isolated from dogs and cats. These strains show in vitro susceptibility to furazolidone as well as gentamicin, neomycin, clindamycin, and tetracycline. However, resistance in many strains to various tetracyclines and kanamycin is caused by plasmids that confer resistance and are transmissible within C. jejuni serotypes. Antimicrobial agents that are usually ineffective include penicillin, ampicillin, polymyxin B, trimethoprim, and vancomycin. In vitro resistance also develops to metronidazole and sulfadimethoxine.183 Many Campylobacter strains produce β-lactamase, which accounts for the resistance to penicillin and ampicillin. Amoxicillin plus clavulanate appears to be effective in treating campylobacteriosis in humans.243 Before therapy is instituted, isolation and susceptibility tests should be done. Some animals continue to shed the organism despite antibacterial therapy. Quinolone antibacterials may be useful in eliminating C. jejuni and C. coli in asymptomatic carriers, but drug resistance to this class of antibacterials has severely impeded their use to treat Campylobacter infections.54 Interestingly, in a human case-control study, therapy with a proton pump inhibitor, omeprazole, doubles the risk of acquiring Campylobacter enteritis.419 The natural resistance imparted by gastric acid secretion has also been observed with other enteropathogens (see Salmonellosis). It is recognized that C. jejuni-coli is a leading cause of enteric disease in people and that puppies and kittens can serve as sources of infection for humans. The infectious dose of C. jejuni for humans is as low as a few hundred organisms. The disease is often severe in people and in addition to diarrhea may be characterized by vomiting, fever, and abdominal discomfort. Usually the incriminated pets have been suffering from diarrhea and have been recently acquired from pet stores or kennels. However, asymptomatic dogs and cats can also be a source of infection to people. Results of a survey conducted in Seattle, Washington, indicated that 6% of sporadic C. jejuni infections were linked to exposure to diarrheic kittens.19 Another study reported that 30% of the cases in university students were associated with healthy cats.166 However, the major risk factor for acquiring C. jejuni enteritis is the consumption of raw or undercooked meat, particularly chicken.17,166,207,563,661 In human infections associated with pets, findings of molecular diagnostic techniques have demonstrated the isolation of the same C. jejuni strain in both the pets and their owners.101,663 Therefore, veterinary practitioners should alert owners of the zoonotic implication of Campylobacter infection for other household members and stress the importance of exercising appropriate hygienic measures, especially when pets have diarrhea. An additional zoonotic risk is the development of Guillain-Barré syndrome, a demyelinating polyradiculoneuritis in people, which may occur after infection with C. jejuni. The antigens of peripheral nerves share epitopes with lipopolysaccharides of these bacteria.408,440 C. upsaliensis has been isolated from a human with bloody diarrhea as well as his pet dog, which also had diarrhea. The plasmid profile of the two strains were identical, suggesting that C. upsaliensis infection may also be a zoonosis.223 C. upsaliensis was isolated from the blood and fetoplacental tissue of a woman with diarrhea who was 18 weeks pregnant and had been in contact with a cat that had a similar strain of C. upsaliensis isolated from its feces, also suggesting zoonotic transmission.239 Others have also reported C. upsaliensis gastroenteritis in humans who have had contact with pets.457 Human and canine isolates of C. upsaliensis have distinct ribotypes and plasmid profiles, suggesting that host-specific genotype differences may exist among strains of C. upsaliensis.566 However, these analyses or other molecular fingerprinting techniques must be used on suspected pet-associated C. upsaliensis zoonoses before the definitive transmission can be defined. Campylobacter spp. and other enteropathogen infections have been prevalent in pets used in human health settings or as guide assistance dogs.235,357,357 The increased popularity of using dogs in hospital- or hospice-based settings as a recognized method of therapy has resulted in the recommendation for screening of these animals for zoonotic microorganisms including Campylobacter spp.356,360 Arcobacters are closely related to campylobacters and have been associated with enteritis and septicemia in humans.483 Clinically healthy dogs have been found to harbor Arcobacter butzleri or Arcobacter cryaerophilus in either their oral cavity or feces.278 No arcobacters were found in cats; however, the sample population was small. As with campylobacters, the common reservoir species for arcobacters are swine and poultry. Whether dogs or cats are involved in zoonotic transmission of Arcobacter spp. infections to humans is uncertain. Gram-negative, microaerophilic, curved to spiral-shaped bacteria isolated from gastric mucosa of humans and animals have created a great deal of interest because of their causal role in gastric disease.349,618 The genus Helicobacter includes 27 formally named species as well as other unnamed closely related organisms.* Once considered a predominantly sterile organ protected from microbial colonization by low gastric pH, the euchlorhydric stomach is now known to be colonized with gastric bacteria belonging to the genus Helicobacter (Web Table 37-2 and Fig. 37-2), whereas the hypochlorhydric stomach is resistant to their replication. These organisms possess a high level of urease activity that allows them to survive in an acidic environment. The type species Helicobacter pylori colonizes the stomach of 20% to 95% of human adult populations worldwide (see Fig. 37-2, A).226 H. pylori causes persistent, active, chronic gastritis and peptic ulcer disease in people and has been linked to the development of gastric adenocarcinoma and gastric mucosa-associated lymphoma.451 H. pylori has also been isolated from inflamed gastric tissue of cats in a commercial cattery and can experimentally infect cats.252,461 WEB TABLE 37-2 Comparative Features of Gastric Helicobacter Species that Infect Dogs and Cats Other gastric helicobacters have been linked to gastritis in various mammalian hosts.167,257,420,435,666 Many of these infected animals have asymptomatic gastric inflammation. Additional species of gastric Helicobacter have been isolated from stomachs of various mammalian hosts, including dogs (Helicobacter rappini, Helicobacter felis, Helicobacter bizzozeronii, Helicobacter heilmannii-like, Helicobacter salomonis, Helicobacter bilis), cats (H. felis, H. heilmannii-like, Helicobacter pametensis, Helicobacter baculiformis sp. nov.),31 ferrets (Helicobacter mustelae), hamsters (Helicobacter aurati), cheetahs (Helicobacter acinonychis), dolphins, whales, harp seals (Helicobacter cetorum), and nonhuman primates (H. heilmannii-like). (See Web Table 37-2 and Fig. 37-2 for a summary of the gastric helicobacters infecting dogs and cats.) Historically, Helicobacter spp. in dogs and cats have been described histologically as gastric spirilla.167 Three morphologically distinct forms have been described in dogs and cats.29a,368,639,640 More than one of these morphologic types of bacteria can be seen in the stomach of one animal. Lockard type 1 is a bacterium with multiple bipolar, sheathed flagella entwined with periplasmic fibers, which appear to cover the entire surface of the organism (see Fig. 37-2, B). A similar organism was isolated from aborted ovine fetuses and was classified as Flexispira rappini.320,439 This bacterium, reclassified as a Helicobacter species based on 16S RNA sequencing, has been isolated from the feces of asymptomatic mice, dogs, and cats as well as from the stomachs of dogs.368,520 H. bilis, a member of the Flexispira taxa associated with hepatitis and IBD in mice, has also been cultured from the stomachs of dogs. Lockard type 2 also has periplasmic fibers, but they are most sparsely distributed on the organism and can appear singly or in groups of two, three, or four. This organism has been cultured from the feline stomach and has been named H. felis (see Fig. 37-2, C).456 The third type of organisms, called gastrospirilla, are the bacteria most commonly found in mammalian stomachs. These are also very tightly spiraled, but they have no periplasmic fibrils. On the basis of morphologic characteristics, it appears that the Lockard type 2 organism primarily is restricted to cats and dogs, whereas type 3 has been seen in cats, dogs, nonhuman primates, cheetahs, swine, and humans. The type 3 bacterium has been given various names in different hosts, including Gastrospirillum hominis and H. heilmannii (see Fig. 37-2, D).255,551 Gastric helicobacters H. bizzozeronii and H. salomonis, H. felis, and Helicobacter cynogastricus242 isolated from dogs have been formally named, based on defined biochemical and genetic analysis. H. bizzozeronii (see Fig. 37-2, E) has also been cultured from the inflamed gastric tissue of humans.18 By EM this organism lacks periplasmic fibers, in contrast to H. felis. This morphologic distinction is confounded with the observation of two gastric Helicobacter-like organisms (GHLOs). These GHLOs from dog stomachs lacking periplasmic fibers had genetic analysis compatible with H. felis.130 H. salomonis (see Fig. 37-2, F) is a gastric helicobacter with unique molecular characteristics that has been isolated from dogs.255,290 In cats, H. felis, H. pylori, H. pametensis (first isolated and characterized in birds), and H. heilmannii have been isolated or identified in gastric tissues. H. baculiformis, a novel species, has also been isolated from the gastric mucosa of a clinically healthy cat.31 Although infected animals and humans mount a significant systemic IgG response to gastric organisms, the antibodies are not protective, and the organisms persist in the mucous layer or closely adhere to the gastric epithelium, protected from the gastric acidic milieu. The mechanisms of transmission are poorly understood. Gastric helicobacters have specific enteric tissue tropism and colonize only gastric and not intestinal epithelium. Fecal-oral transmission has been suggested, and gastric helicobacters have been isolated from feces of animals and humans.452 H. mustelae was isolated from feces of ferrets, particularly when ferrets had drug-induced hypochlorhydria.194 H. pylori has been isolated from feces of children from a third-world country as well as from infected adults from a more developed country.312,599 Gastric helicobacters have been isolated from the environment in surface waters,262 likely a result of fecal contamination. Indirect transmission in this manner might result in more widespread dissemination. One concern is that helicobacters are more resistant than coliforms to chlorination. Oral-oral transmission is more likely and is supported by clinical observations of humans being infected by exposure to gastric secretions,452 isolation of H. pylori from dental plaque and tissue, and nosocomial infection from improper disinfection of gastric pH probes and endoscopic equipment. Similar transmission routes are also probable for gastric Helicobacter in animals. Helicobacter spp. DNA has also been detected in the oral cavities of dogs.489 Vomitus containing gastric Helicobacter is another likely source for transmission.152 In pups, experimentally inoculated gastric helicobacters have been acquired during the lactation period, and puppies have infected each other.254 Cats naturally infected with H. pylori were screened by culture and PCR for the organism in salivary secretions, gastric juices, gastric tissues, and feces.195 H. pylori was cultured, from salivary secretions (50%) and gastric fluid samples (91%), from cats. A PCR product specific for an H. pylori surface protein was amplified in 42% of feline dental plaque specimens and 80% of feline fecal specimens. Isolation of H. pylori from feline mucosal secretions suggests a zoonotic risk exists from personnel handling of H. pylori–infected cats in vivaria.252 In comparison, H. pylori infection was not detected in stray cats,133 which may reflect an anthroponosis in the closed commercial cattery where H. pylori was present in 100% of the animals. Additional studies using molecular, cultural, and histologic techniques are needed to ascertain whether H. pylori naturally colonizes dogs and cats. The prevalence of gastric Helicobacter spp. infections in clinically healthy colony-raised dogs and cats in high population densities routinely approaches 100%, indicating the organisms’ unique ability to colonize the stomachs of numerous hosts selectively and efficiently.539 In laboratory-reared beagles, H. felis and other spiral helicobacters were observed in high numbers in the gastric glands of the fundic-pyloric junction and the cardia. These organisms were associated with lymphoid hyperplasia and parietal cell degeneration.264,368 In pet dogs and cats a series of gastric biopsy samples were examined for the presence of GHLOs.268 GHLOs were observed in 82% of dogs and 76% of cats; the bacteria were present in the mucus of foveolar epithelia, gastric pits, and parietal cells. More lymphoid follicles are found in the stomachs of older random-source cats with high numbers of GHLOs in their gastric mucosae than in younger cats with lower numbers of GHLOs.444 A high prevalence of infection was observed, in association with histologic gastric inflammation, in 89.2% of stray cats.27a Helicobacter spp. have been found in the oral cavity and stomach of stray cats; however, the species in the oral cavity did not always correlate with that found in the stomach.584a Chronic or chronic active gastritis resulting from oral inoculation of Helicobacter species has been experimentally produced in humans, germ-free pigs and dogs, specific-pathogen free cats, mice, and nonhuman primates with H. pylori; in ferrets with H. mustelae; in kittens with Helicobacter acinonychis and H. heilmannii and in germ-free dogs, specific-pathogen free dogs, mice, and rats with H. felis.167 H. pylori–associated gastritis in humans consists of polymorphonuclear cells as well as mononuclear cell infiltrates and is classified as active chronic gastritis. Persistent H. pylori infections in humans (particularly children) and in the domestic cat also are often characterized by lymphoid aggregates and gastric lymphoid follicles.174,253 Inflammation and associated lymphoid follicles were primarily located in the antrum, which corresponded to the heaviest concentration of H. pylori.252 Ultrastructurally, the organisms were numerous in the gastric mucus, were less frequently adhered tightly to gastric epithelia, and formed pedestals between bacterial membranes and the epithelial microvilli.252 Gastric lymphoid elements had been considered a normal histologic finding in dogs and cats. However, experimental and clinical evidence suggests that they are the result of host responses to Helicobacter antigens.542 The presence of GHLOs is often associated with a reduction in mucus content of surface epithelia, occasional intraepithelial leukocytes, and some degenerating glands. The gastritis observed in dogs and cats with non–H. pylori helicobacters is generally mild. Eosinophils in gastric mucosae of animals can also be a major component of the inflammation, particularly in the acute phase of the infection.174,350,350 Neutrophils and eosinophilic infiltrates, interepithelial globule leukocytes, epithelial dysplasia, and upregulation of certain mucosal proinflammatory interleukin (IL)-1β and IL-8, and interferon-γ are noted in H. pylori–infected cats but not in uninfected control cats.142,541,541 The degree of GHLO colonization and lymphoid follicles correlated well in cats but not in dogs. If the number of GHLOs in dogs was classified as high, then lymphoid follicles were more likely to be present. In high-grade GHLO infections, glandular degeneration was more pronounced in cats than dogs.268,585,585 In other dogs343 the severity of gastritis correlated with infection but not with the clinical sign of vomiting. In other studies,129 100% of laboratory and shelter dogs and 67% of pet dogs were colonized with GHLOs that were morphologically consistent with H. heilmannii or H. felis. Regardless of the colonization intensity, all dogs had mild to moderate gastritis, and H. pylori was not isolated. Experimentally, H. felis infection in dogs for 26 weeks did not correlate with number of H. felis observed and degree of inflammation or number of affected lymphoid follicles.538 Furthermore, the gastric secretory axis was not perturbed in H. felis–infected dogs or dogs naturally infected with GHLOs.540 Competitive inhibition, whereby the colonization by one organism suppresses proliferation of other helicobacters, may occur in gastric helicobacteriosis. This phenomenon has also been suggested to account for the rare occurrence of concurrent infection in humans and nonhuman primates with large gastric spiral organisms and H. pylori.119,120,263,349,571 Experimental inoculation of the feline H. pylori strain into naïve cats without gastric infection caused by GHLOs confirmed that H. pylori produces a persistent gastritis identical to that noted in cats naturally infected with H. pylori.174 H. pylori was isolated on serial biopsies and at necropsy 7 months after inoculation from all cats.174 Additional studies are required to ascertain whether duodenal and gastric ulcers have an infectious component in dogs and cats.178
Enteric Bacterial Infections
Campylobacter Infections
Etiology
Campylobacter Organism
Morphologic and Distinguishing Features
Hosts
Clinical Features
C. jejuni; C. coli
Slender, curved, motile rods; found singularly, in pairs, or in chains with three to five spirals; curved S- or gull-shaped; single, polar, nonsheathed flagellum; C. coli: hippurate negative
Dogs, cats, ferrets, cattle
Neonates—diarrhea
Adults—asymptomatic
Humans
Diarrhea, systemic manifestations
C. upsaliensis
Catalase negative
Dogs, cats
Asymptomatic
Humans
Diarrhea, abscess, placental damage
C. lari
Grows at 42° C
Dogs
Asymptomatic
Humans
Bacteremia, urinary tract infections
C. helveticus
Catalase negative
Cats
Asymptomatic
Epidemiology
Pathogenesis
Clinical Findings
Dog
Cat
Diagnosis
Microscopic Examination
Cultural Identification
Serologic Testing
Molecular Identification of Campylobacter species
Pathologic Findings
Therapy
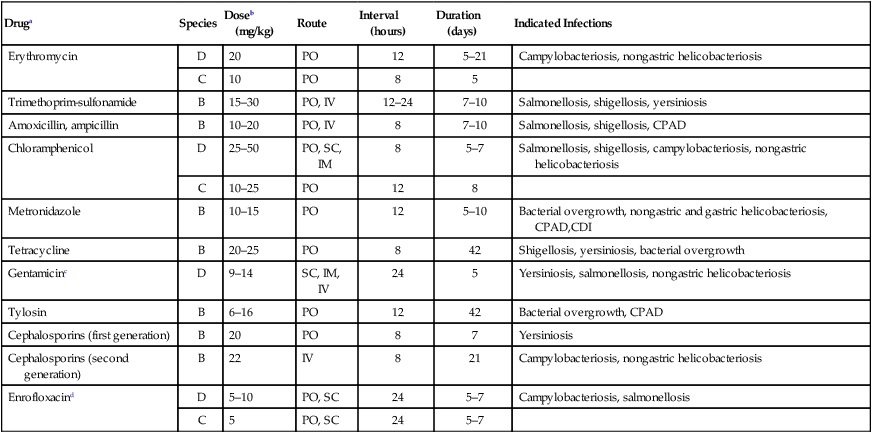
Druga
Species
Doseb (mg/kg)
Route
Interval (hours)
Duration (days)
Indicated Infections
Erythromycin
D
20
PO
12
5–21
Campylobacteriosis, nongastric helicobacteriosis
C
10
PO
8
5
Trimethoprim-sulfonamide
B
15–30
PO, IV
12–24
7–10
Salmonellosis, shigellosis, yersiniosis
Amoxicillin, ampicillin
B
10–20
PO, IV
8
7–10
Salmonellosis, shigellosis, CPAD
Chloramphenicol
D
25–50
PO, SC, IM
8
5–7
Salmonellosis, shigellosis, campylobacteriosis, nongastric helicobacteriosis
C
10–25
PO
12
8
Metronidazole
B
10–15
PO
12
5–10
Bacterial overgrowth, nongastric and gastric helicobacteriosis, CPAD,CDI
Tetracycline
B
20–25
PO
8
42
Shigellosis, yersiniosis, bacterial overgrowth
Gentamicinc
D
9–14
SC, IM, IV
24
5
Yersiniosis, salmonellosis, nongastric helicobacteriosis
Tylosin
B
6–16
PO
12
42
Bacterial overgrowth, CPAD
Cephalosporins (first generation)
B
20
PO
8
7
Yersiniosis
Cephalosporins (second generation)
B
22
IV
8
21
Campylobacteriosis, nongastric helicobacteriosis
Enrofloxacind
D
5–10
PO, SC
24
5–7
Campylobacteriosis, salmonellosis
C
5
PO, SC
24
5–7
Public Health Considerations
Arcobacter Infections
Gastric Helicobacter Infections
Etiology
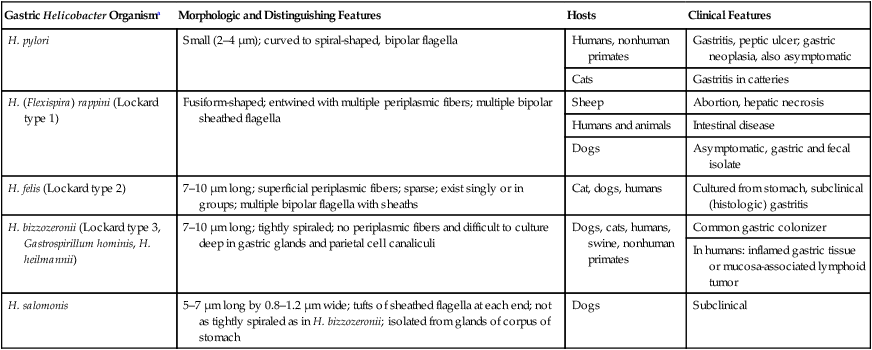
Gastric Helicobacter Organisma
Morphologic and Distinguishing Features
Hosts
Clinical Features
H. pylori
Small (2–4 µm); curved to spiral-shaped, bipolar flagella
Humans, nonhuman primates
Gastritis, peptic ulcer; gastric neoplasia, also asymptomatic
Cats
Gastritis in catteries
H. (Flexispira) rappini (Lockard type 1)
Fusiform-shaped; entwined with multiple periplasmic fibers; multiple bipolar sheathed flagella
Sheep
Abortion, hepatic necrosis
Humans and animals
Intestinal disease
Dogs
Asymptomatic, gastric and fecal isolate
H. felis (Lockard type 2)
7–10 µm long; superficial periplasmic fibers; sparse; exist singly or in groups; multiple bipolar flagella with sheaths
Cat, dogs, humans
Cultured from stomach, subclinical (histologic) gastritis
H. bizzozeronii (Lockard type 3, Gastrospirillum hominis, H. heilmannii)
7–10 µm long; tightly spiraled; no periplasmic fibers and difficult to culture deep in gastric glands and parietal cell canaliculi
Dogs, cats, humans, swine, nonhuman primates
Common gastric colonizer
In humans: inflamed gastric tissue or mucosa-associated lymphoid tumor
H. salomonis
5–7 µm long by 0.8–1.2 µm wide; tufts of sheathed flagella at each end; not as tightly spiraled as in H. bizzozeronii; isolated from glands of corpus of stomach
Dogs
Subclinical
Epidemiology
Pathogenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Enteric Bacterial Infections