Chapter 16 Thoracoscopy
Thoracoscopy Introduction: Indications, Instrumentation, Techniques, Biopsy Procedures, and Complications
www.tamssmallanimalendoscopy.com
Indications
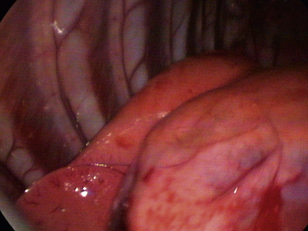
One of the benefits of thoracoscopy over thoracotomy is that all areas accessible by the approach normally taken for the disease process can be evaluated with enhanced visualization and decreased morbidity. Not only can all areas of the thorax be seen, but magnification and lighting are also greatly enhanced, increasing the probability of identifying small lesions (Figure 16-1). Multiple samples of abnormalities may be obtained for histopathologic examination; small or early lesions may be easily identified by thoracoscopy.
Instrumentation
The equipment required for thoracoscopy differs little from that required for laparoscopy except there is no need for an insufflator (see Figure 15-1). The chest is a rigid wall, the lungs collapse when ports are placed, and further collapse is possible with selective bronchial intubation or bronchial blockade, which may make insufflation unnecessary.
Any rigid endoscope may be used for thoracoscopy; however, most surgeons prefer to use a 30-degree endoscope and have a 0-degree endoscope available. The 0-degree endoscope is best suited for the surgeon early in training and for initial experience with thoracoscopy, as the view is easiest to understand, straight on, and simple to process. However, the 0-degree endoscope requires the surgeon to lever against the ribs to view the entire thoracic cavity. This problem can be alleviated by use of the 30-degree endoscope, which has a field of view that is angled from the long axis of the endoscope. The offset angle allows complete examination of the thoracic cavity without levering against adjacent ribs (Figure 16-2). The surgeon can “look around corners” with the angled endoscope, place less torque on the ribs adjacent to the port site, and decrease the risk of damage to the endoscope. By rotating the 30-degree endoscope, the surgeon views a wider area than with the 0-degree endoscope. Rotation of a 0-degree endoscope causes no change in the field of view.
Ports used for thoracoscopic surgery are usually open rather than valved, as insufflation is rarely used or recommended for thoracoscopy. Open ports allow for rapid exchange of instruments and decrease trauma to the adjacent thoracic wall. Some procedures have been done in an assisted fashion; in those cases ports are removed, a port opening is extended to a minithoracotomy, and instruments used in open surgery complete the procedure. If insufflation of the thorax is desired for further atelectasis, valved ports may be used; however, pulmonary changes in patient status often offset the benefits of insufflation. Ports that are soft and flexible (Figure 16-3) decrease the pressure applied to adjacent soft tissue and ribs and may decrease the perioperative pain associated with nerve compression by rigid ports against ribs. They are useful at intercostal sites and can be sutured in place to decrease dislodgement when instruments are changed.
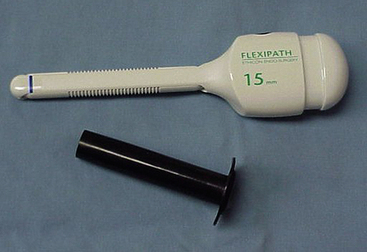
Figure 16-3 Flexible endoscopic port that can be transected at a length that matches the depth of the thoracic wall.
(Photograph © 2010 University of Georgia Research Foundation, Inc.)
Flexible ports (Flexipath, Ethicon, Inc., Somerville, N.J.) may also be cut to size to fit the patient better and may be resterilized for repeated use in similarly sized patients. Friction may cause problems that inhibit instrument or endoscope movement within the flexible port. Water-soluble lubricant will decrease friction, but the endoscope end must be avoided to minimize problems with visualization.
Laparoscopic ports may be used for thoracoscopy but may be longer than desired. Smooth laparoscopic ports can dislodge easily when placed between ribs. Traditional laparoscopic ports are also often supplied with valves that are not necessary for thoracoscopy and sharp trocars. There is a great risk of organ trauma when sharp trocar–port assembly insertion is used for thoracoscopy, and it is more likely to damage pulmonary parenchyma. Open placement, establishment of a pneumothorax, and placement under direct visualization may not be enough to decrease the risk of trauma. Replacement of the sharp trocar with a blunt trocar is recommended if laparoscopic ports are used for thoracoscopy. Threaded ports are helpful, as they remain in place during instrument changes for thoracoscopic evaluation. The paraxiphoid approach requires a longer port; using a long, threaded port for slow, controlled placement with a 0-degree endoscope allows the surgeon to visualize each structure penetrated, and exact placement in the pleural space is then made possible. Excessively deep port placement can be avoided as can tissue trauma. Threaded ports may also be used intercostally and are more secure than smooth laparoscopic ports (see Figure 15-6).
Diagnostic Procedures
The instruments used for the majority of diagnostic thoracoscopy include a palpation probe, which is used for palpation, manipulation, and measurement of organs and lesions; cup and punch biopsy forceps to sample the liver, lymph nodes, lesions, and the pancreas; straight and curved grasping forceps for manipulation and minor dissection required for sampling; and scissors for dissection and sample excision (see Figure 15-1). Samples of pleura, mediastinum, pericardium, and lymph nodes may be obtained with these instruments. Addition of a preformed ligature loop allows sampling of lung margins. An aspiration–irrigation port can be used to evacuate large volumes of pleural or pericardial fluid and to irrigate a field of dissection. Alternatively, a small Poole suction tip or a feeding tube can be inserted through a port to obtain fluid samples and drain large volumes of fluid. Spinal needles can be inserted through the thoracic wall to obtain pericardial fluid samples under direct, endoscopic visualization. Sampling for cytologic evaluation, bacterial culture and susceptibility testing, fungal culture, and histopathologic examination should be considered during every thoracoscopic exploration.
Therapeutic Procedures
More instrumentation will be required to perform different therapeutic interventions. Straight and curved dissectors are needed to dissect structures within the thorax. Lymph node removal, mass removal, ligation of the ligamentum arteriosum, ligation of the thoracic duct, and pericardectomy are performed with several types of dissecting forceps and scissors. Different tissue types require different forceps for grasping and retraction. Fine tissues do not require large, toothed forceps, but more aggressive grasping forceps are needed to grasp and manipulate tougher tissue, such as the pericardium and other connective tissues within the mediastinum. A fan retractor is required to retract lung away from the site of dissection for many procedures and is vital for dissection of the pulmonary hilus, ligamentum arteriosum, and PDA to avoid pulmonary trauma and postoperative pneumothorax (see Figure 15-4). Lung biopsy is greatly facilitated by the application of pretied suture loops. Clip appliers are very useful for hemostasis and ligation of the thoracic duct and are required for PDA ligation. Even more advanced procedures, such as assisted or endoscopic partial or complete pneumolobectomy, require endoscopic gastrointestinal anastomosis (GIA) or thoracoabdominal (TA) stapling devices. Removal of specimens larger than those collected with biopsy forceps is best achieved with specimen retrieval bags so that port site metastasis or infection is avoided (see Figure 15-102, Figure 15-103). Bags can be purchased or made from those available in stores.
The majority of the instruments used in diagnostic or therapeutic procedures (e.g., grasping forceps, scissors, and dissectors) can be connected to electrocautery or radiosurgical devices. The insulated shaft allows for monopolar use. Specially designed bipolar forceps and cutting devices are also available. More specialized devices for incision and/or sealing of vascular structures include harmonic scissors and vessel-sealing devices. These devices make mass excision, pericardectomy, and advanced dissection such as that required for mediastinal debridement in cases of pyothorax far simpler; in addition, they maintain visualization while minimizing collateral tissue damage. The more advanced the procedure, the more advanced the instrumentation usually used.
Patient Preparation and Positioning
Securely fasten the positioned patient to the operative table, and be certain the position is appropriate for the intended or most complicated procedure. The patient may be positioned in dorsal, lateral, or sternal recumbency, as required for the procedure. Consider whether the patient’s position will require alteration intraoperatively. Changing the position may allow gravity to provide improved exposure of the desired site. Different equipment can be used, such as tilting tables or tabletop additions that allow for the position to be angled or changed. Please review Chapter 15 for the baseball concept for surgeon and trocar positioning in relationship to the operative site and monitor (see Figure 15-12), as well as the use of tilt tables (see Figure 15-26Figure 15-27). Take care to secure the patient well if a table or table-top devices are used so that dramatic positional changes with contamination during surgery are avoided. Rapid conversion to an open approach should be considered when the patient is being secured to the operating table. Be certain that assistants can release limb ties as rapidly as possible if the position of the patient must change for conversion to open thoracotomy.
Anesthesia
General anesthesia and mechanical ventilation are standard for thoracoscopy in veterinary medicine. Physical status, systemic compromise, and ventilatory ability of the patient should be considered, and the anesthetic technique should be tailored to the patient’s needs. Pneumothorax is required during thoracoscopy, and when both lungs are mechanically ventilated, a decreased partial pressure of oxygen (PaO2) and increased partial pressure of carbon dioxide (PaCO2) occurs. These changes are attributed to ventilation-perfusion mismatching but are not severe enough to result in clinical compromise in healthy dogs. A decrease in total peripheral vascular resistance occurs secondary to hypercapnia but is not severe and does not cause complications in healthy experimental dogs. Clinical cases may not show normal ventilation or perfusion, and changes in tidal volume, inspiratory pressure, and ventilatory rate should be based on changes in monitoring variables. Therefore, adequate monitoring equipment must be used to detect changes and to adjust the ventilation as required by the patient.
Therapeutic Procedures
The use of a video camera and video monitor is vital in therapeutic procedures. An assistant directs the endoscope while the surgeon performs the procedure. Without a video camera and monitor, this would be impossible. Concurrent viewing by all members of the operating and anesthetic team allows correlation of the patient’s progress with the progress of the procedure, linking the entire team together, maximizing the quality of patient care.
Port Placement
Choose an initial port site that will allow examination of the entire pleural space and triangulation of subsequent ports for biopsy or therapeutic intervention (see Figure 15-12). In a lateral approach, make a skin incision slightly larger than the diameter of the port. Bluntly dissect the musculature of the thoracic wall until the parietal pleura is penetrated. The size of the thoracotomy should be large enough to allow easy placement of a blunt trocar and port. Examine the entire hemithorax before placement of subsequent ports, which should be done under direct visualization with the endoscope (see Figure 16-20).
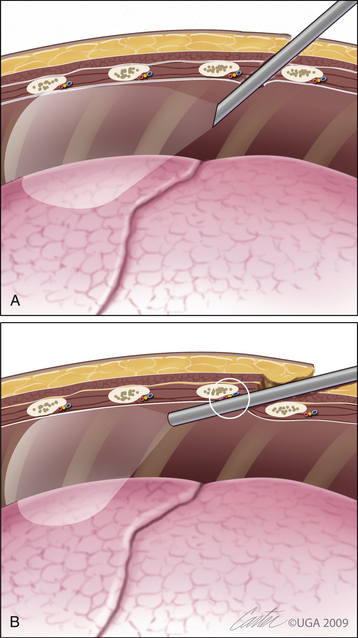
The paraxiphoid port is often placed first in patients undergoing thoracoscopy in a dorsal position (see Figure 16-19). Place the port with concurrent visualization through a 0-degree endoscope within the lumen of the port. A threaded port is ideal for such placement. Make an incision through the skin to one side of the base of the xiphoid process between it and the costal arch. Port placement is easiest if it is introduced into the ipsilateral hemithorax. It is important to make the incision larger than the diameter of the threaded port to avoid gathering of the skin and subcutis on port advancement. Place the port and slowly advance it, using a clockwise motion, toward the ipsilateral thoracic wall and somewhat dorsally, to ensure entry into the pleural space. Introduce a 0-degree endoscope as soon as the port engages the musculature. Advance the port under endoscopic guidance until it penetrates the diaphragm and diaphragmatic pleura. Advance slightly beyond the pleura to allow uninhibited insertion and removal of the endoscope and other instruments through the port. The initial evaluation of the chest through the paraxiphoid port is limited to the hemithorax entered (Figure 16-4). Fenestrate the mediastinum through a port placed in the ipsilateral intercostal space to allow bilateral exploration (Figure 16-5). The mediastinum will often adhere to the contralateral lungs until bilateral pneumothorax has been established. Fenestrate the mediastinum using two pairs of grasping forceps placed in a poorly vascular section; a fatty mediastinum may require stabilization with grasping forceps and an incision with scissors. Use electrocautery, radiosurgery, or vascular sealers to avoid any hemorrhaging that would obscure further visualization.
Place other ports after exploration of the chest. Their placement depends on the expected procedures to be done. Triangulation is required for accurate and easy operation and should be planned. Subsequent portal placement should be done under thoracoscopic visualization. Identify the port site by placing pressure on the intercostal space with concurrent endoscopic visualization. Minithoracotomies and placement of ports with blunt obturators have been described. Each port can be used for examination or passage of operating instruments. Portals placed for lateral thoracic procedures may be placed anywhere from dorsal to ventral, as long as the site is visualized with the endoscope. Consider the movement of the pulmonary parenchyma, and place ports ventrally enough to avoid the pulmonary parenchyma during instrument entry and manipulation. Care should be taken to avoid the internal thoracic arteries, which should be easy to visualize. Operating portals should be spaced far apart so that interference between instruments and the endoscope is avoided. Use a 30-degree endoscope to obtain the widest variety of fields of view and the least risk of instrument interference.
Biopsy Techniques
Pleural Biopsy
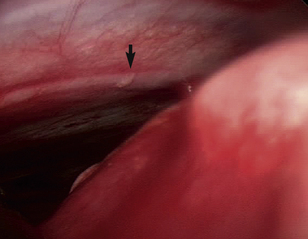
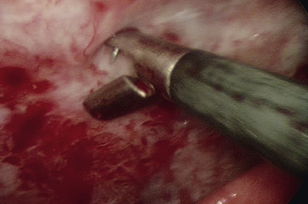
Pleural biopsy can be done during any thoracoscopic exploratory surgery. Avoid the intercostal vessels (Figure 16-6) and nerves located at the caudal aspects of the ribs to minimize the risk of life-threatening hemorrhaging. Insert cup biopsy forceps under thoracoscopic visualization, and use them to palpate the ribs cranial and caudal to the intended site of pleural biopsy to avoid the neurovascular structures. This method should avoid the nerves and vessels even in cases in which the thickened parietal pleura obscures their view. Open the forceps, and take samples from the parietal pleura; significant force is not routinely required (Figure 16-7). Should significant hemorrhaging occur, electrocautery, radiosurgery, vascular clip application, or open thoracotomy may be required. Take multiple samples of the parietal pleura, including multiple abnormal and apparently normal sites in an attempt to differentiate reactive mesothelium from mesothelioma or metastatic neoplasia.
Lung
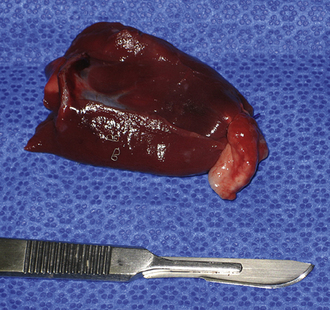
Use pretied loop ligatures to sample the distal 2 cm of lung lobes. Introduce the pretied loop ligature and grasping forceps into the thoracic cavity. Grasp the edge of the lung through the loop ligature, and elevate it while tightening the ligature during exhalation. Tightening the loop with the lung fully inflated increases the risk of pleural and parenchymal damage. Stabilize the lung and ligature, and excise the area to be sampled with Metzenbaum scissors; last, transect the excess suture with hook scissors and remove it from the thorax (Figure 16-8).
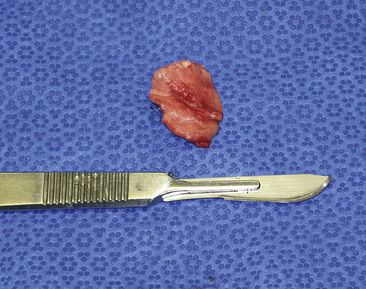
Figure 16-8 A pulmonary biopsy sample taken with a loop ligature.
(Photograph © 2010 University of Georgia Research Foundation, Inc.)
Endoscopic stapling devices can be used to obtain larger lung biopsy specimens and may be fired twice to perform wedge excisions of lung (see Chapter 11; Figure 11-3) (Figure 16-9).
Postoperative Care
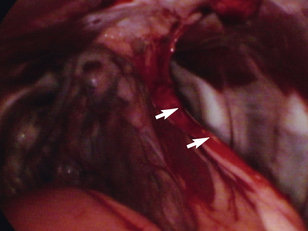
Place a thoracostomy tube in all cases except those in which solid tissue biopsy was achieved with minimal hemorrhaging. Place the tube in a normal fashion under endoscopic visualization (Figure 16-10); grasping forceps can be used to adjust tube location within the pleural space. Do not utilize one of the port sites, and be certain that the subcutaneous tunnel is appropriate. Secure the tube with a purse-string and Roman sandal–type of suture pattern, and wrap the thorax to avoid tube dislodgement. Ensure that the thoracic bandage does not inhibit ventilation.
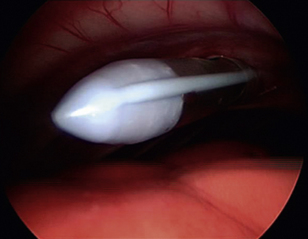
Figure 16-10 Thoracoscopic visualization of the tip of a thoracostomy tube during placement.
(Photograph © 2010 University of Georgia Research Foundation, Inc.)
Complications
Hemorrhaging or pneumothorax after thoracoscopy is similar to that after open thoracotomy and may require treatment. Tumor seeding of port sites after thoracoscopy has been reported in human patients and in dogs. Care should be taken to avoid this complication, but a diagnosis is not always known before thoracoscopy; widespread neoplasia, such as mesothelioma, can affect port sites despite proper handling of tissues. Use endoscopic retrieval bags for removing samples after biopsy or mass excision; however, port site metastasis can still occur, despite this practice.
Normal Findings
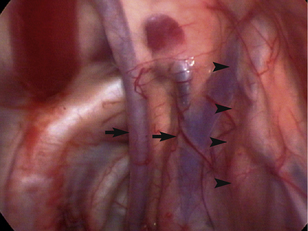
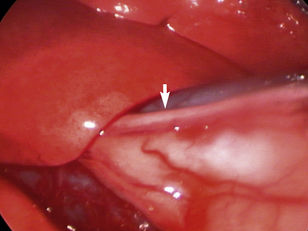
Many structures within the normal thorax are easily identified. The thoracic wall is easily viewed through the pleura, which is quite thin in healthy patients. Ribs, costochondral junctions, intercostals vessels, and nerves should be easy to identify (see Figures 16-4 and 16-6). Depending on the positioning of the patient, many other structures should be seen. If the patient is in dorsal recumbency, all of the following should be evaluated: sternebrae, internal thoracic vessels, pericardium, sternal lymph nodes, lungs, and diaphragm. The normal ventral mediastinum is thin and interlaced with fat and may be readily visible in the central portion of the ventral thorax on initial examination; however, it may be adhering to the visceral surface of the contralateral lung lobes (see Figure 16-4). On fenestrating or opening the mediastinum, the surgeon should examine the lungs on both sides of the chest; they should be pink and easily retracted. The pericardium can easily be seen, and the phrenic nerves should be visible at the base of the heart surrounded by pericardial fat (Figure 16-11). Rotate the patient or grasp the pericardium to examine the phrenic nerves bilaterally. The heart should be visible through the translucent pericardium. View the pulmonary vasculature by retracting the lung away from the heart with atraumatic instruments such as a palpation probe or fan retractor. Thymic tissue is readily apparent in the mediastinum of young patients. Hilar and dorsal structures may be more readily viewed via a lateral approach. Dorsal structures such as the sympathetic trunk, thoracic duct(s), azygos vein, mainstem bronchi, and hilar lymph nodes may be identified with significant retraction or changes in patient positioning.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree