Chapter 15 Laparoscopy
Laparoscopic Surgery Introduction: Indications, Instrumentation, Techniques, and Complications
www.tamssmallanimalendoscopy.com
The history of laparoscopy in small animals is similar to that in human surgery, except that widespread veterinary adoption has lagged 15 to 20 years. Laparoscopy was first performed in a dog in 1902, before clinical laparoscopy in people. Although laparoscopy was used routinely by a few clinicians, widespread human application was delayed, probably related to the need for better and more cost-effective endoscopes, improved light sources, and camera and imaging processing capabilities.1 The first reported liver biopsy was done in 1972.2 This liver biopsy technique continues to be the most commonly indicated and performed laparoscopic technique in dogs and cats. In people and small animals, the other historical delay was that indications for laparoscopy were restricted to diagnosis of organ disease.
Using endoscopy to treat disease has markedly increased the indications and client enthusiasm for endoscopy. In people, two treatment approaches, laparoscopic cholecystectomy and arthroscopy, led the transition from traditional to minimally invasive (endoscopic) surgery. More than 500,000 cholecystectomies are performed annually in the United States. In 1987, Mouret developed a laparoscopic approach to gallbladder removal.3 Multiple laparoscopic modifications were soon developed, and within 2 years, the majority of cholecystectomies done in the United States were performed laparoscopically.4 The advantages of the minimally invasive cholecystectomy include smaller incisions, shorter hospitalization, and a more rapid return to work. There appears to have been resistance to this new laparoscopic approach from some traditional surgeons in the United States, especially those in academia. The widespread demand for laparoscopic cholecystectomy appears to have been more in response to patient request than any study documenting less pain, shorter hospitalizations, and other positive outcomes. Patients wanted small incisions and perceived reduced trauma. When audiences at veterinary endoscopy courses are asked if they would prefer a traditional surgery at a cheaper cost as compared with a more expensive minimally invasive surgery, people routinely respond that they do not want the traditional and would insist on the endoscopic procedure. Many pet owners agree that their four-legged family members should have the same advantages of minimally invasive surgery as they would want for themselves.
Indications for Laparoscopy
Nearly all of the organ biopsy specimens taken by traditional laparotomy can be obtained by laparoscopy (Box 15-1).5–7 Specific indications and techniques for biopsies of the liver, spleen, kidneys, pancreas, prostate, gastrointestinal tube, lymph nodes, and masses are included in the section by David Twedt. Organs are better viewed laparoscopically than during traditional surgery. Images and movies can also be recorded. These can be used to monitor disease severity and to communicate with the client and veterinary colleagues. The primary missing examination tool is digital palpation. The ability to palpate organs, such as in the liver and spleen, during laparotomy may be overrated. Some appreciation of organ palpation for lesion localization can be obtained with the use of palpation probes and ultrasonographic transducers during laparoscopy. The diagnostic value of laparoscopy for histologic definition and staging of cancer is high and is a laparoscopic application that is underappreciated. Samples taken for histologic, microbiologic, and toxicologic examination are much larger and more precisely sampled than those obtained with ultrasonography. When compared with obtaining these samples by traditional laparotomy, laparoscopy markedly reduces the surgical time and trauma. Many clients will positively respond to the clinician’s recommendation for a liver biopsy if it can be done through two small incisions, whereas they frequently reject biopsy recommendations when the only option is an open laparotomy. Some efficient surgeons will find that their initial laparoscopic liver biopsies seem longer than laparotomies that they have performed hundreds of times. Motivated endoscopic surgical novices can quickly become proficient with equipment setup and will find their operative times reduced below that of their laparotomy times. The time savings is that required to open and close a midline laparotomy incision.
Laparoscopic treatments, which have developed during the past decade, markedly expand the types and numbers of small animal procedures; in addition, the number of indications that can be treated with laparoscopy has expanded (Box 15-2). Most procedures on this list have been documented in publications, and some, such as laparoscopic-assisted gastropexy and laparoscopic-assisted cystoscopic calculi removal, have been performed in hundreds of patients. The gastropexy, cystoscopic-assisted calculi removal, and spay procedures are well accepted and have been taught to hundreds of veterinarians during “hands-on” endoscopy courses. Specific procedures are presented in the treatment sections.
This treatment list is being expanded almost daily as more surgeons and practitioners become comfortable with endoscopy and are presented with the challenges of managing diseased patients. When a new procedure is contemplated, it should be one that is doable by traditional technique and has a reasonable chance of being accomplished in a similar, minimally invasive fashion. The laparoscopic procedure should not be contraindicated for that patient, such as repair of a diaphragmatic hernia. Another contraindication is the lack of surgical proficiency, for example, in performing endoscopic knot tying. The client must be informed and be prepared to accept that the procedure might not be accomplished endoscopically but may require conversion to the traditional laparotomy technique. An advantage of attempting a minimally invasive surgery in a client-owned patient is that conversion can be done with the only disadvantage being the cost of instrumentation and increased surgery and anesthesia time. The consent for endoscopic surgery should always include a phrase such as, “Dr. _____ is given permission to convert to a traditional or open procedure if Dr. _______ deems conversion to be best for patient care.” Development of some techniques requires the use of research subjects. An example is laparoscopic-assisted gastropexy in which tests of breaking strength were needed to confirm an effective adhesion between the abdominal wall and antrum. Procedures such as cystoscopic calculi removal and resection of intestinal tumors were first performed in client-owned patients. If the endoscopic surgery had failed, permission to convert to the traditional procedure was obtained from the client before surgery. These clients were most appreciative, and their pets benefited from the minimally invasive procedure. When considering conversion, the surgeon must periodically be introspective about the progress of the endoscopic surgery and be willing to accept that an alternative approach might be best. Input may also be provided by another surgeon, the anesthetist, or a colleague. Some endoscopic surgeons set an arbitrary time limit for minimally invasive surgery before conversion to traditional surgery.
Basic Laparoscopic Instrumentation
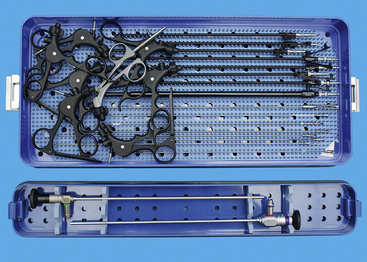
Laparoscopy packs should include trocar-cannula units, scopes, and endoscopic surgical tools (Box 15-3). Endoscopic instruments tend to be the same as used for traditional surgery except that they are long and have a narrow shaft suitable for passing through the cannulae. These instruments include forceps, scissors, retractors, needle holders, electrocautery and other energy devices, and suction/lavage instruments. The trocar-cannula units and endoscopic tools may be disposable or reusable. Most of the reusable tools are stainless steel, and many veterinarians prefer them for their cost-effectiveness. Reusable instruments can be properly cleaned and sterilized in a steam autoclave. This section will include specific instruments for a starter system. The reader is referred to Chapter 1 on instrumentation for more details.
BOX 15-3 List of Laparoscopic Equipment for Simple Diagnostic Studies and Laparoscopic-Assisted Treatments
The essential laparoscope is a 5-mm, 0-degree endoscope, which can be used in most cats and dogs. A 5-mm, 30-degree laparoscope should be the next scope purchased, as it is preferred for examination of cranial and caudal regions of the abdomen, the adrenal glands, the sides of organs, and thoracoscopy. The 30-degree imaging provides the option of seeing “around corners” and is especially useful for examining all sides of liver lobes. It can be very useful when working in confined area. These areas may be so confining that cannulae must be placed closely together, which makes it difficult to use the 0-degree scope for surgical manipulations. A 2.7-mm diameter, 15-cm long, 30-degree scope can be useful for laparoscopy in cats, small dogs, and small exotic animals. When the 2.7-mm scope is used, a 3-mm trocar-cannula reduces the size of the initial incision. The 2.7-mm scope should be restricted to smaller patients because its smaller size reduces the viewing field and its fewer light fibers reduce illumination. Ten-millimeter scopes provide more light and a larger image. They are bulky and larger than ideal for most dogs but should be considered for larger patients. Their bulk also increases durability, as they are less prone to being bent. Bent scopes produce a halo in their image, which should be avoided to decrease scope injury. I have tried to use an operating laparoscope, in which an endoscopic instrument is passed through a channel of the large-diameter operating laparoscope; however, I prefer not to use it because the disadvantage is the loss of depth perception in the absence of triangulation. Some surgeons like the 10-mm operating laparoscope system for one-port ovariectomy or ovariohysterectomy (see the “Laparoscopic Ovariohysterectomy” section of this chapter).
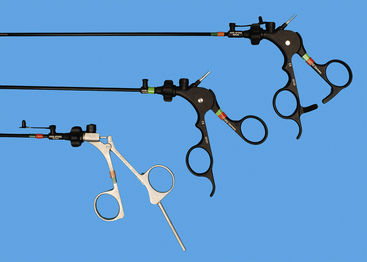
Recommended endoscopic instruments for dogs and cats are 5 mm, except for a 10-mm Duval forceps (see Box 15-3, Figures 15-1 and 15-2). The 10-mm forceps is used to grasp larger and thick walled organs, such as the gastric antrum for gastropexy. Initially the Babcock forceps was recommended, but the slightly more aggressive Duval tips secure the antrum better and the double action produces a wider grasp. There are considerable differences between various Babcock and Duval forceps tips, even within the same endoscopic equipment companies’ product lines. The tips should grasp firmly but should not penetrate viscus walls. Five-millimeter instruments for the starter pack should include biopsy forceps (cup and punch), scissors (dissecting), forceps (Babcock, dissecting), and a palpation probe. Handles are interchangeable, but the locking type are used for grasping devices and needle drivers, whereas the nonlocking handles are used for scissors and biopsy devices (see Figure 15-2). The scissors and dissecting forceps can have an attachment electrode for monopolar electrocautery. A variety of lengths are available, but the shaft and sheath must be the same length. For simplicity, lengths for the initial pack should be the same to reduce confusion for beginning operators. As the endoscopic surgeon becomes more confident, additional 5-mm instruments include another dissecting forceps with a right-angle tip, suction/lavage probe, knot-tying forceps (see Chapter 13), suture scissors (Figure 15-3), and fan retractor (Figure 15-4 and Box 15-4). Ancillary equipment is also needed and should be accumulated as necessary and as the practice expands (Box 15-5). Although bipolar instruments are available from endoscopy companies, widely used bipolar instruments are also available from companies selling power generators. Some seal large vessels and others strictly coagulate. Some have the capability to dissect and cut tissue.

Figure 15-3 As the endoscopic surgeon develops his or her skills, a suture scissors (right) will be needed in addition to dissecting scissors (left). Suture scissors are used to cut sutures after placement of pre-tied endoscopic loops or when endoscopic knot tying is performed (see Chapter 13).
(Photograph by Chris Herron © 2010 University of Georgia Research Foundation, Inc.)
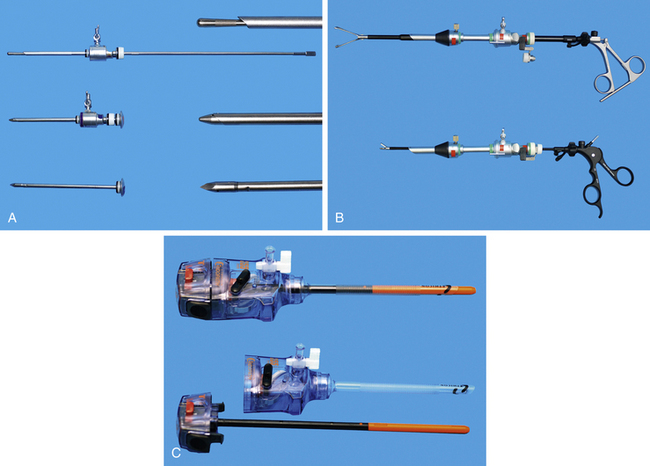
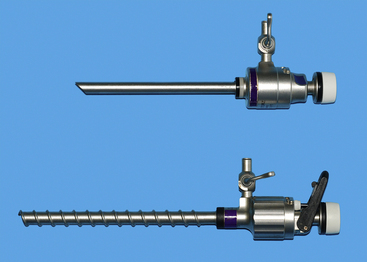
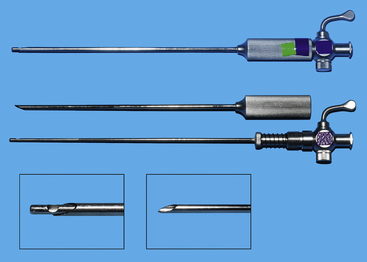
Cannulae must be appropriate for passage of 5-mm instruments; usually the outer cannula diameter is 5.5 to 6 mm. The initial pack should include at least three such cannulae: two should have sharp trocars and the third should be blunt (Figure 15-5, A). A cannula for a 10-mm Duval forceps is needed for laparoscopic gastropexy (Figure 15-5, B). The type of cannula is a personal preference. The most common have a smooth external sheath, which has a tendency to slide in or out of the abdominal trocar site. Some smooth trocars have a method for securing the trocar to the incision. An excellent but more expensive option is disposable trocar-cannulae (Figure 15-5, C). An Olive occluding device can be used to aid in sealing leaks about the trocar site and in securing the trocar to the trocar site. A popular alternative to smooth-surface trocar-cannulae is screw-in cannulae, which are retained well in the incision (Figure 15-6). Their disadvantage is that they can be more traumatic to place. Some clinicians prefer to have both, using the smooth for quick procedures, such as liver biopsies and grasping of organs to be delivered to the surface for laparoscopic-assisted procedures, and the screw-in cannula for spays. A smooth trocar for the 10-mm Duval forceps should be included for its use in the gastropexy. A reducer valve should be available to reduce the opening for a 10-mm instrument to one for a 5-mm instrument. In this way biopsy of an organ through an 11-mm trocar can be performed before a gastropexy in which the same cannula is used for a 10-mm Duval forceps. Reusable trocar-cannulae may have replaceable parts, such as the rubber grommet. Reusable cannulae can have either an automatic or manual flap-valve. Disposable trocar-cannulae tend to have sharper tips, are lighter, and are more self-retaining. These advantages make them the preference of most physicians, but their typical silastic valves make them very difficult to clean and sterilize. Reuse will also produce tears in the silastic valves. Insufflation tubing can be purchased from the endoscopic manufacturer. Tubing can be resterilized but can have connectors and filters that are deformed during steam sterilization. Filters are typically discarded by veterinarians after their initial use. Other acceptable insufflation tubing is silastic tubing with a Luer-Lok connector that is purchased from an endoscopy manufacturer. Suction line tubing has also been used for insufflation tubing.
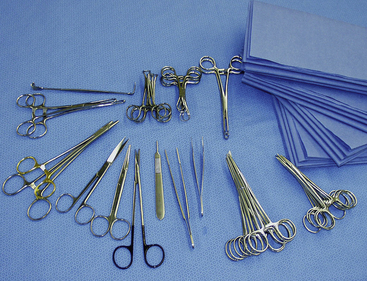
Surgical equipment complementary to endoscopic instruments are similar to either a spay or general surgery pack (Figures 15-7 and 15-8). Typical instruments include tissue forceps (DeBakey and closure tissue forceps), approximately 8 mosquito hemostats, approximately 6 Kelly hemostats, scissors (Metzenbaum, Mayo, and suture), 2 high-quality needle holders, 12 towel clamps, 2 nonperforating Lorna towel clamps, and large draping. Draping for laparoscopy and thoracoscopy should provide a large opening in a large aseptic field and cover the entire patient and operating table. Because light and camera cables are attached to critical and expensive cameras and laparoscopes, these cables and the insufflation tubing must be secured to prevent damage and contamination. There should be stable, draped areas to place these instruments during the procedure. A large, draped back table works best to securely hold all equipment.
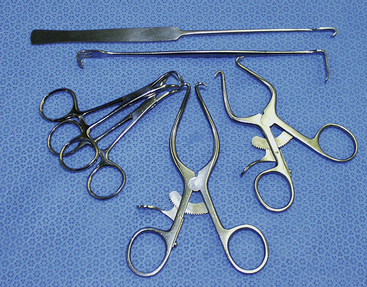
Figure 15-8 Retractors commonly used for laparoscopy.
(Photograph © 2010 University of Georgia Research Foundation, Inc.)
Principles of aseptic surgery are essential, even though the incisions are small. Surgical instruments are either steam autoclaved or gas sterilized. Reusable endoscopic instruments can usually be steam autoclaved. It is critical to dissemble the take-apart instruments (Clickline) and to clean them in an enzymatic bath according to the manufacturer’s guidelines. I prefer to pack these instruments disassembled and have the surgical team aseptically reassemble them during the surgery. The camera, scopes, and light cables are usually gas sterilized with the use of either Sterad or ethylene oxide. Disinfection with glutaraldehyde (Cidex) or the use of a camera sleeve can also be used for the camera and light cable. If glutaraldehyde is used, the instruments must be thoroughly flushed with sterile fluids. Considering the expense of endoscopic equipment and the potential for injury, I strongly recommend that endoscopic boxes be used for pack preparation, sterilization, and storage (Figure 15-9). If several endoscopic surgery cases are scheduled for the same day, planning is required to rotate and sterilize instruments. The trocar-cannulae and endoscopic instruments can be steam autoclaved. Some clinicians have extra light cables, trocar-cannulae, and energy equipment and use a camera sleeve instead of glutaraldehyde disinfection for the camera and light cable (Figure 15-10). Although many laparoscopes are rated for steam autoclave, care must be taken to strictly follow the manufacturer’s guidelines. An option that avoids reusing steam-autoclaved laparoscopes or glutaraldehyde disinfection is rotating a 5-mm, 0-degree with a 5-mm, 30-degree laparoscope and a 2.7-mm endoscope.
Recording images and movies digitally is considered essential by many clinicians. These are useful for documenting and monitoring disease. Clients, veterinary colleagues, hospital staff members, and referring veterinarians appreciate being able to view diseases and treatments that have been performed. It is very useful to compare endoscopic images with other imaging findings, for example, those seen on radiographs, ultrasonograms, and computerized tomography scans. Many clinicians and hospitals use images and movies as a critical part of their endoscopic marketing.
Accessory Endoscopic Instruments
Several energy and stapling instruments are useful, even essential, when more advanced laparoscopic procedures are performed. These are covered in Chapter 11. Electrocautery, vessel-sealing devices, and other power equipment reduce hemorrhaging while tissue is cut. These techniques are critical when the clinician performs an ovariectomy, ovariohysterectomy, adrenalectomy, and other vascular dissections. Clips and endoscopic knot tying can be applied to larger vessels, such as renal vessels, as another means of hemorrhage control. Gastrointestinal resections and anastomoses can be performed with the use of stapling equipment during laparoscopy.
Patient Evaluation and Preparation
The same patient evaluation performed before laparotomy should also be done before laparoscopy. All patients must have complete historical and physical examinations. Evaluations should include a complete blood count, platelet count, serum chemistry profile, and urinalysis. Some practices may opt for more modest laboratory studies for young, healthy dogs and cats undergoing elective procedures such as cryptorchidectomy, ovariectomy, ovariohysterectomy, and gastropexy. Patients requiring biopsies of the kidney, liver, or masses should also have coagulation studies. Depending on the disease condition, clinical judgment can indicate the need for more complete organ function and imaging studies (e.g., ultrasonography, radiography, computed tomography, and contrast studies).
Anesthesia and Monitoring
General anesthesia with positive pressure ventilation should be given for nearly all laparoscopies.8–10 Some clinicians prefer sedation plus local anesthesia for quick liver biopsies. Considering the safety of general anesthetic drugs, the monitoring capability in veterinary practices, and the stability gained by general anesthesia, I strongly prefer general anesthesia.
Minimally invasive surgery has been welcomed by anesthesiologists for its ability to reduce postoperative pain and respiratory problems.8 Several pain studies have identified less pain after minimally invasive surgery as compared with traditional or maximal surgery. Despite these pain studies, I ensure pain reduction by continued practice of analgesia during and after surgery.
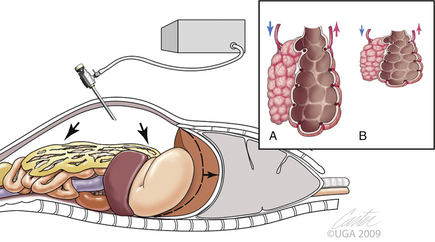
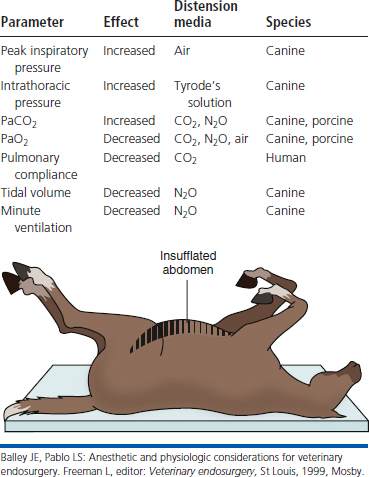
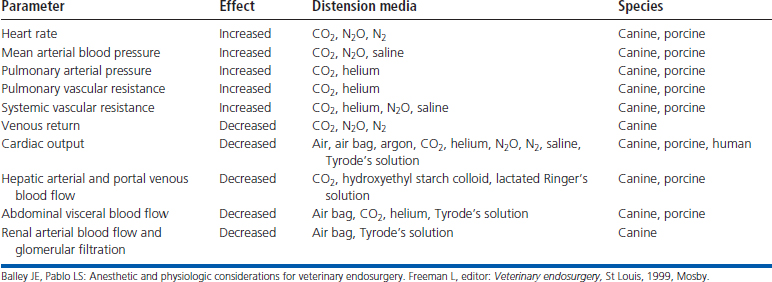
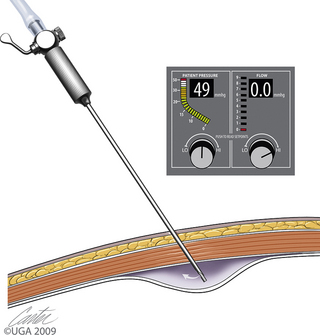
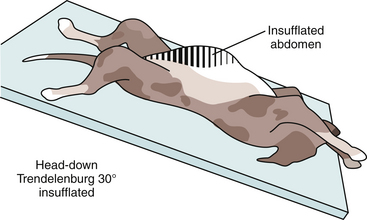
Carbon dioxide distension of the abdominal cavity dramatically affects ventilation and venous return (Figure 15-11).8–10 Pressure pushing cranially on the diaphragm decreases extrapulmonary compliance, tidal volume, and minute volume. Intrathoracic pressure, peak inspiratory pressures, and most vascular pressures and resistances are increased (Tables 15-1 and 15-2). Unless corrected, the arterial carbon dioxide tension increases and the arterial oxygen tension decreases. These changes are exacerbated when the patient’s head is down (Trendelenburg position) (Table 15-3). Higher abdominal pressures and greater degrees of table tilt increase the pulmonary compromise. Some clinicians use the “Rule of 15’s” as safety guidelines for intraabdominal pressure and degree of Trendelenburg tilt. The high positive pressure pneumoperitoneum requires positive pressure ventilation. The initial settings are typically 12 ventilations per minute; one third of the time is for inspiration, and the remaining time permitted is for expiration. Peak inspiratory pressure is typically 12 mm Hg. It is best if ventilation is adjusted according to the arterial or end-tidal carbon dioxide tensions. Ventilation can be provided by a mechanical ventilator or by hand. The critical factor is monitoring of cardiopulmonary measures.
Table 15-3 Summary of the Physiologic Effects of Elevated Intraabdominal Pressures and Head-Down Tilt
| Parameter | Effect |
|---|---|
| Impedance of lung and chest wall | Increased |
| Peak inspiratory pressure | Increased |
| Deadspace ventilation | Increased |
| PaCO2 | Increased |
| PaO2 | Decreased |
| Pulmonary compliance | Decreased |
| Functional residual capacity | Decreased |
| Vital capacity | Decreased |
| Mean arterial blood pressure | Increased |
| Left ventricular pressure, dp/dt and end-systolic wall stress | Increased |
| Systemic vascular resistance | Increased |
| Intracranial pressure | Increased |
| Cardiac output | Decreased |
 | |
Balley JE, Pablo LS: Anesthetic and physiologic considerations for veterinary endosurgery. Freeman L, editor: Veterinary endosurgery, St Louis, 1999, Mosby.
Venous return is decreased by intraabdominal insufflations (see Table 15-2). Arterial pressure can also be decreased by positive pressure ventilation. Consequently, arterial pressure should be monitored. Decreased arterial blood pressure treatments may include increased intravenous fluids, reduction of the anesthetic concentration, and administration of cardiovascular drugs.
Laparoscopic Techniques
Trocar-Cannula Placement
Laparoscopy produces excellent two-dimensional viewing. This better image is produced by better lighting, magnification, and scope/camera optics. Depth perception is poor, similar to viewing with a single eye, and can be contrasted with the depth perception achieved with two eyes used during traditional surgery. When instruments seen through a laparoscope are used, triangulation of the scope with the instrument(s) provides depth perception. Thus, trocar-cannula placement must be such that triangulation is produced.11,12 For simple biopsies, such as liver biopsies, only two trocar-cannulae are needed. One is for the laparoscope, and the other is for the biopsy device. A similar approach is used for laparoscopic-assisted procedures in which the second trocar is for a grasping instrument to extract an organ to the trocar incision. When the trocar-cannula incision is lengthened, the organ can be extracted sufficiently for such procedures as a gastropexy, intestinal biopsy, intestinal resection and anastomosis for tumors and foreign bodies, or removal of cystic calculi with a cystoscope. Most true laparoscopic operative procedures require at least three trocars: one for the scope and the other two for instruments to dissect, cut, suture, tie knots, apply clips, and apply energy for hemorrhage control.
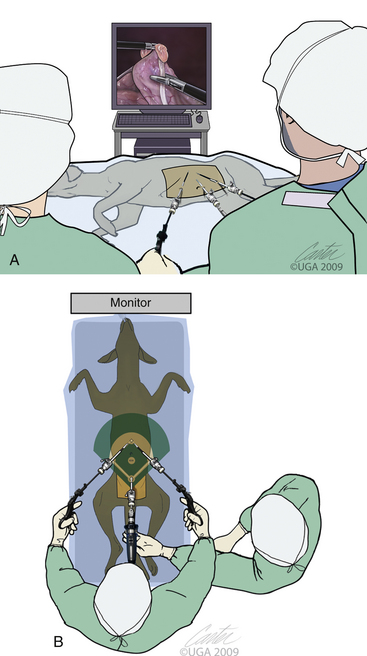
The classic arrangement for these three trocars is a baseball infield (Figures 15-12 and 15-13). The catcher position is the laparoscope trocar and the operative instrument trocars are going along the first and third base paths toward second base. The laparoscopic image has triangulation and depth perception of both instruments as they manipulate the target site (second base). This “baseball” approach must be considered with each surgery. Consideration given to instrumentation orientation also includes the sagittal and transverse planes (Figure 15-14). Failure to do so produces instrument motions seen on the monitor that are moving in the opposite direction as those same instruments are moving within the abdomen (Figure 15-15). Violating the baseball concept can be done by an experienced endoscopist if necessary, but it is tedious, fatiguing, and headache-producing. Endoscopic surgery, like all surgeries, must be accomplished as simply as possible with the surgeon in a comfortable position. When two surgeons are operating from opposite sides of the patient, the baseball field concept and comfort for both surgeons can be best maintained by both if a second monitor is provided for the assistant surgeon (Figure 15-16). The image for the second monitor is taken from the first. It is frequently helpful to move the scope from one trocar to another to improve imaging and performance of the surgery.
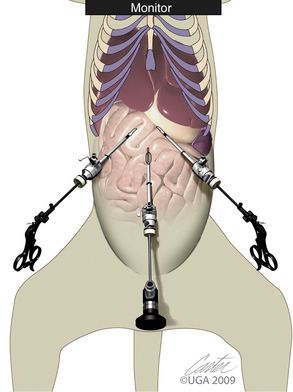
Figure 15-13 Close-up of baseball field concept with patient and instruments.
(Art by Kip Carter © 2010 University of Georgia Research Foundation, Inc.)
Triangulation of an endoscopic instrument in relationship to the laparoscope provides some depth perception. Depth perception is lost if the instrument and scope are parallel or an operating laparoscope is used. Some clinicians consider this arrangement as “dueling swords” because the scope and instrument are coming from the same direction and are headed toward the same target. In addition to depth perception, there is a limited range of instrument motion in relation to each other. When using an instrument running parallel to the scope or when using an operating scope, the clinician can gain appreciation of the instrument and target organ by advancing the endoscopic instrument gently toward the target organ. Depth is determined when the instrument touches the organ. Triangulation during proper baseball field positioning can also be assisted by the use of a 30-degree laparoscope. The viewing should be such that the 30-degree viewing is directed toward the operative site taking full advantage of the side view. The baseball concept also applies in the sagittal plane. Poor trocar-cannula positioning can produce challenges for less experienced endoscopic surgeons attempting laparoscopic-assisted cystostomy to remove calculi when the calculi are cranial to the cystoscopic site. Other deep structures have also been a problem in this vertical plane when the trocar-cannula sites are placed too close to the monitor with respect to the organ.
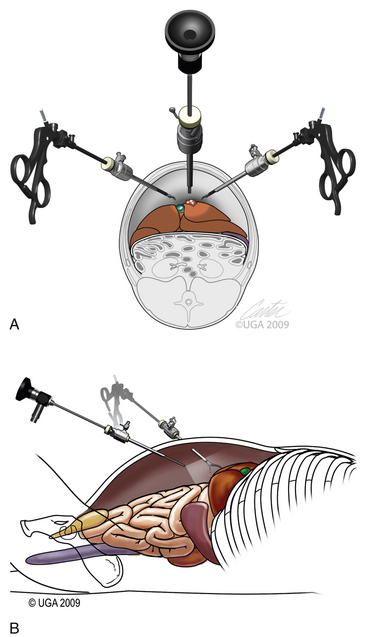
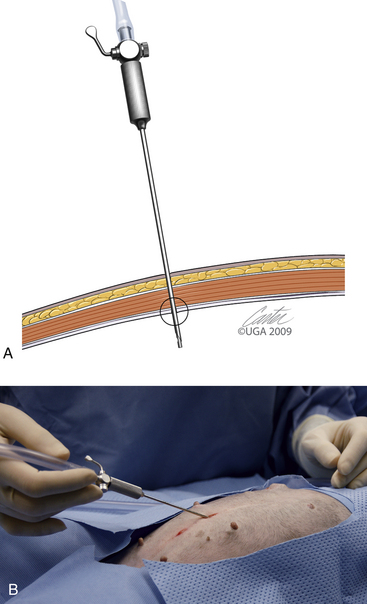
The first trocar for insufflation and initial laparoscope placement can be inserted with at least three different techniques. The two classic methods use a Veress needle or a Hasson technique. The Veress needle consists of a sharp outer trocar and a blunt inner stylet within the trocar (Figure 15-17). A skin incision is made and the needle advanced through the abdominal wall. Once the sharp needle penetrates the peritoneal cavity, a spring advances the blunt stylet forward within the needle to protect abdominal structures from being cut. Some clinicians describe a “pop” as the peritoneum is penetrated (Figure 15-18, A). Once inside the abdominal wall, the needle is gently rotated to attempt to validate that the complete abdominal wall has been penetrated. A small amount of fluid should easily flow through the Veress needle. The clinician should attempt aspiration to check for bowel contents and blood. A drop of saline placed on the needle hub should flow gently into the abdominal cavity when the wall is lifted. Insufflation is then started (Figure 15-18, B). An advantage of the Veress technique is that it can be done on the side or midline, in contrast to the Hasson technique, which is routinely done on the ventral midline. A common veterinary practice is to place the Veress needle on the right side with the patient in left lateral recumbency. This position can be used for liver biopsies, pancreatic biopsies, and enterostomy tube placement. The disadvantages are the potential damage of abdominal organs, insufflation of the space between the abdominal musculature and the peritoneum (Figure 15-19), fatal air embolism,13 and insufflation of omental or falciform fat.
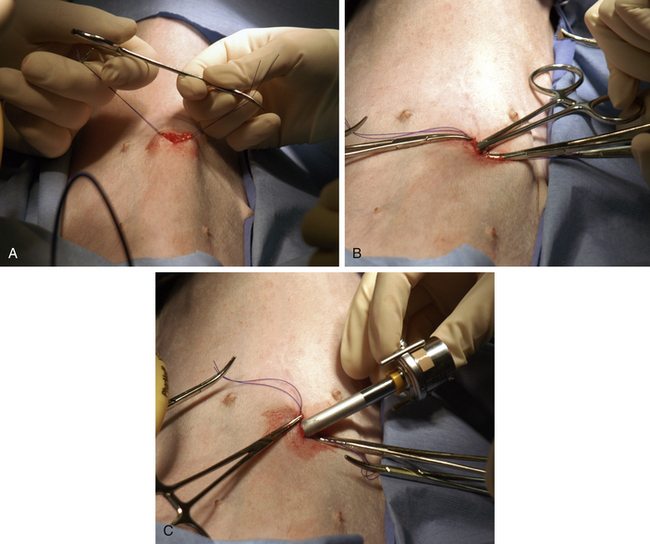
The Hasson technique is done using a 5-mm cannula with a blunt-tipped trocar (see Figure 15-5, A); however, direct observation is first provided by performance of a minilaparotomy (Figure 15-20). If a blunt-tipped trocar is not available, a palpation probe can be placed into the peritoneal cavity and the cannula can be advanced over it. Placement should be on the midline, and the same anatomic precision is required as when a midline spay incision is made. The animal should be in strict dorsal recumbency. The skin and subcutaneous fat are incised, approximately 3 cm caudal to the umbilicus, 2.5 to 3 times as long as the trocar is wide. This length increases with the thickness of the subcutaneous fat. The fat is dissected from the external sheath approximately 1 cm laterally. Traction sutures are placed 5 mm to 1 cm lateral on each side of the intended minilaparotomy. These sutures are used to lift the abdominal midline for the midline incision and can be used for securing the trocar and providing a seal if the abdominal incision is too large. The incision through the midline into the abdomen is made with a No. 15 blade. The incision through the midline should be short to ensure a snug fit of the abdominal wall around the trocar. The intraperitoneal position is confirmed by placing a blunt curved forceps (mosquito or small Kelly) into the peritoneal cavity and rotating the forceps (Figure 15-20, B). The hemostat should spin freely. The trocar is then forced through the minilaparotomy incision. The initial thrust is in a right cranial direction to avoid the spleen and umbilical fat. The trocar is rotated axially as it is advanced and directed just inside the abdominal wall once the peritoneum is penetrated. The trocar is also rotated to reconfirm an intraperitoneal location. Care is taken to avoid abdominal organ injury throughout trocar placement. Insufflation is then started. The advantage is that intraabdominal trauma may be reduced as compared with the use of a Veress needle and the surgeon can be confident that insufflation is being delivered intraperitoneally. The disadvantages are that placement is on the ventral midline and the initial skin incision is larger than with the Veress needle. If the minilaparotomy is larger than the cannula, carbon dioxide will leak (Figure 15-21). If this occurs, the abdominal pressure will be below the preset pressure. When this occurs, an encircling mattress suture can be placed or a dilating “Olive” can be added to the trocar (Figure 15-22). Temporary occlusion of leakage can be accomplished with moist gauze held tightly around the trocar. Another alternative is also performed on the ventral midline (Figure 15-23). The skin incision can be smaller than with the Hasson but must be long enough to define the midline. Sutures are used as in the Hasson technique. After the initial incision, a 12F round-tipped urinary catheter is passed into the intraperitoneal space and insufflation is started. After the peritoneal cavity is distended, the trocar is inserted. This technique works well with a screw-in trocar.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree