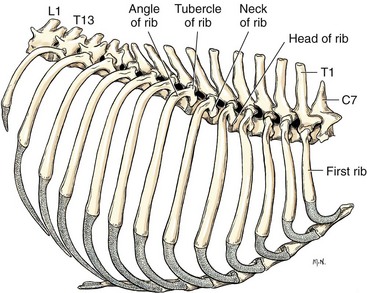
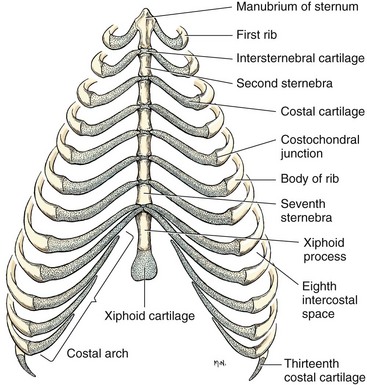
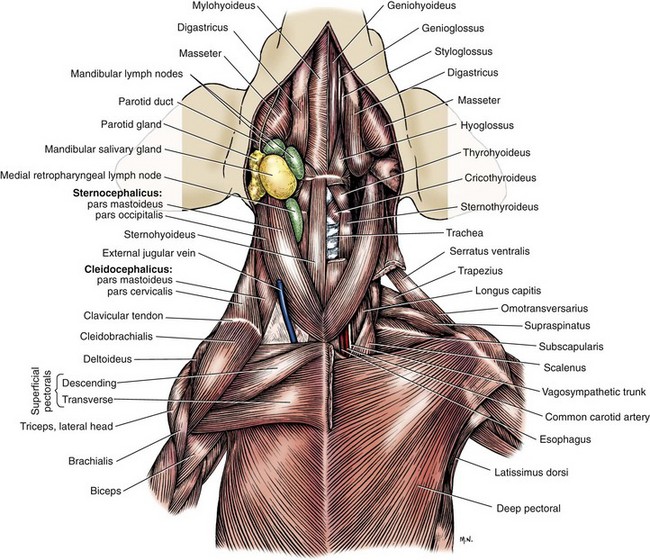
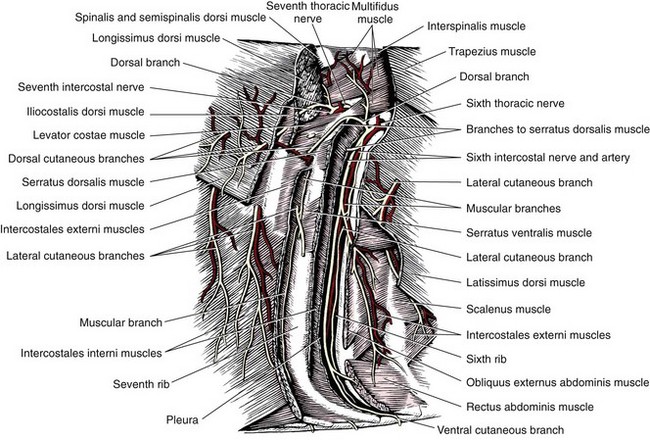
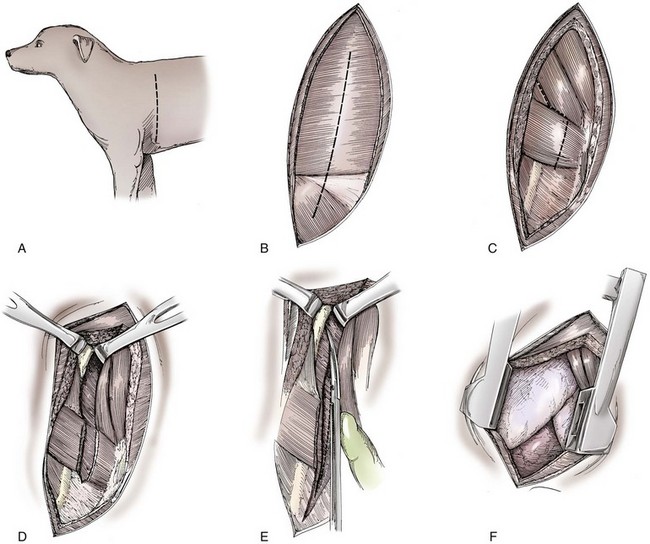
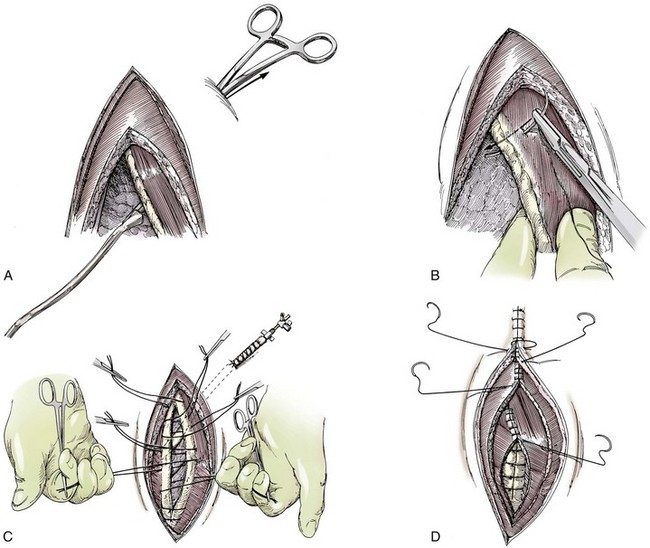
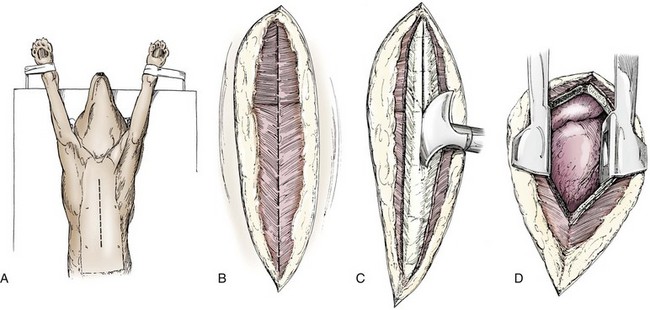
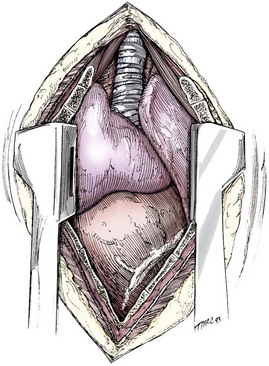
Chapter 104 The complex musculoskeletal structure of the thorax facilitates complete resection of tumors with adequate margins. Natural tissue planes allow separation of muscle bellies to reduce postoperative morbidity, and anatomic landmarks allow intraoperative delivery of local anesthesia.12 The caudal extent of the thoracic cavity is marked by the diaphragm, consisting of two muscular crura that arise by tendons from the fourth lumbar vertebra and have muscular attachments to the lumbar vertebrae, ribs, and sternum. The diaphragmatic crurae are joined by a central tendinous portion. Nerves, vessels, and the esophagus enter or leave the thorax through the esophageal and aortic hiatii and the caval foramen.12 Skin of the thoracic wall possesses some anatomic features that may be useful for reconstruction. Skin of the dorsolateral thorax is supplied mainly by the thoracodorsal artery, which arises from the subscapular artery and supplies the latissimus dorsi muscle and the skin. Flaps that can be based on the thoracodorsal artery include the thoracodorsal axial pattern flap and a composite musculocutaneous flap incorporating the latissimus dorsi muscle. This flap is particularly useful because it provides a means of reestablishing integrity of the thoracic wall as well as skin coverage.12 The double-layered elbow fold may be separated from the upper foreleg and unfolded to produce a subdermal plexus flap capable of closing defects of the sternum or lateral thorax. Dogs and cats have 13 thoracic vertebrae, 13 ribs, and 9 sternebrae (Figures 104-1 and 104-2). Ribs one to nine articulate with the sternebrae via cartilaginous extensions from the costochondral junctions. The sternebrae are connected to one another by fibrocartilage. The cranialmost sternebra, the manubrium, provides the point of attachment for the sternocephalicus muscle. The caudalmost sternebra, the xiphoid, is positioned dorsal to the linea alba and ventral to the falciform ligament. The xiphoid does not have any direct muscular attachments other than the diaphragm; the fascial connection between the paired rectus abdominus muscles (linea alba) merges with the fascia of the deep pectoral muscle ventral to the xiphoid.12 Muscle groups (Figures 104-3 and 104-4) associated with the thorax include the intrinsic and extrinsic muscles of respiration, muscles of the abdominal wall, and locomotor musculature.12 The locomotor muscles attach the forelimb to the trunk. Most significant for thoracic surgeons are the latissimus dorsi, the serratus ventralis thoracis group, and the superficial and deep pectoral muscles. The latissimus dorsi muscle originates from the lumbodorsal fascia and thoracolumbar vertebrae and converges cranioventrally to insert on the proximal humerus. It draws the scapula and hence the forelimb caudally. The latissimus dorsi muscle may either be divided or elevated to gain access to the intercostal muscles for intercostal thoracotomy. Limb function does not seem to be unduly impaired if this muscle is separated or relocated. Elevation of the muscle has been suggested to cause less postoperative pain but can also reduce surgical access or necessitate more active retraction to maintain surgical exposure. The thoracolumbar attachments of the latissimus dorsi muscle can be incised and the muscle rotated ventrally to close large defects in the thoracic wall. Intercostal nerves (Figure 104-5) arise as ventral branches of the thoracic spinal nerves and pass ventrally along the caudal edge of each rib in association with the intercostal arteries and veins. There are 12 intercostal arteries on each side of the thorax. The first three or four are branches of the thoracic vertebral artery, and the remainder are branches of the aorta. Dorsal branches of the intercostal arteries descend in the intercostal spaces along the caudal borders of ribs 1 through 12. They give off branches dorsally that penetrate the epaxial muscles and ventrally that perforate the intercostal and adjacent muscles to supply cutaneous structures, including the thoracic mammary glands. Ventrally, the intercostal branches anastomose with ventral intercostal branches of the internal thoracic artery. The intercostal arteries can be avoided by incising the intercostal muscles midway between ribs in the dorsal two thirds of the intercostal space; however, small anastomotic branches are often cut ventrally during intercostal thoracotomy. Orientation of the heart and other viscera within the thorax is important for determining the most appropriate surgical approach for various conditions (see Table 105-1). Left intercostal thoracotomy is always used for approaches to the left side of the heart (Figure 104-6). The right atrium, venae cavae, and azygous vein are most accessible via a right lateral thoracotomy, although the cavae can also be identified and tourniquets placed from a ventral and left approach. Median sternotomy provides good visualization of the cranial vena cava from its origin as the brachiocephalic veins to where it enters the right atrium. The right ventricle arcs around the cranioventral border of the heart, and the right ventricular outflow tract and pulmonary artery are best accessed from a left intercostal or a ventral approach. The anterior free wall of the left ventricle is accessible through a left lateral thoracotomy, but the left ventricular apex is most accessible via a transdiaphragmatic approach, which is thus the best approach for insertion of epicardial pacemaker leads. Because the thoracic wall and lungs are functionally linked by negative pleural pressures, total pulmonary compliance is a function of the additive compliance of the thoracic wall and lungs. Abnormalities in total pulmonary compliance may result from changes in either lung or thoracic wall compliance.33 Alterations in thoracic volume after procedures such as thoracic wall resection or advancement of the diaphragm or spontaneous diseases such as pectus excavatum or large thoracic wall tumors have an impact on ventilation and tidal volume. Likewise, changes in lung volume after lung lobe resection or secondary to pathologic conditions such as restrictive pleuritis, chronic emphysema, or pleural effusion have an impact on thoracic volume, limited by the compliance of the thoracic wall. For fourth and fifth intercostal thoracotomy, the skin is incised 2 cm caudal to the scapula from the caudodorsal angle of the scapula to just below the costochondral junction (Figure 104-7). Subcutaneous tissue is incised, and the latissimus dorsi muscle is divided with scissors or electrocautery. Alternatively, the latissimus dorsi may be elevated and displaced by incising its ventral fascial attachments and retracting it dorsally. Neuromuscular paralysis assists by reducing muscle spasm during incision and allowing the ribs to be retracted farther. After the intrathoracic procedure has been completed, the pleural cavity is lavaged and inspected for ongoing hemorrhage or air leakage. The sandbag or drape is removed from beneath the patient’s chest to facilitate rib apposition. Three to four cruciate sutures, or four to six encircling sutures, of 2-0 to 1 polydioxanone suture are placed around the ribs cranial and caudal to the thoracotomy (Figure 104-8). The assistant places traction of one or more of the sutures while the remaining sutures are tied. The aim of the pericostal sutures is to reduce tension on the soft tissue repair rather than immobilize the two ribs completely. The sutures should therefore be tied securely but not overly tightly. Ribs may be fractured during suture tightening or after surgery if pericostal sutures are too tight. By its nature, placement of circumcostal sutures will entrap intercostal nerves. Transcostal sutures have been used in a small case series; results suggest that animals experience less postoperative pain compared with conventional techniques.41 A ventral midline skin incision is made, and the subcutaneous tissue is incised. The pectoral muscles are separated down the midline (Figure 104-9). Careful electrocautery reduces the amount of bleeding encountered from perforating branches of the internal thoracic artery and vein associated with each sternebra. Hemorrhage from the veins, particularly, can be difficult to control. Air embolism is common during sternotomy, and small air bubbles are often seen within the internal thoracic veins after the thorax is opened. The pectoral muscles are separated from their attachments to the ventral surface of the sternum; extending the dissection laterally can result in hemorrhage and is best avoided. After the midline and a small portion of each sternebra to either side have been exposed, a combination of palpation and visualization is used to determine the appropriate line for the sternotomy. Deviation from the midline should be scrupulously avoided. Scoring the sternebrae with a scalpel or electrocautery before osteotomy may be helpful to reduce straying to one side or the other. The sternebrae are sectioned using a reciprocating saw, osteotome, special sternal saw, sternal splitter, or bone cutters. In smaller patients, the reciprocating saw causes less compression of their compliant chest and thus permits a more accurate incision to be made. The sternotomy is commenced at the cranial or caudal end, depending on the objective for the surgery. After the first one or two sternebrae have been sectioned, a Gelpi retractor is inserted into sternal cartilage between the split edges, and the sternum is retracted. This stabilizes the sternum and produces some tension to facilitate extended incision along the midline. It also allows the connective tissue of the ventral thorax to be perforated, thus establishing a pneumothorax to ensure that the heart, lungs, or great blood vessels are not damaged during the incision. The incision may then be easily continued into the caudal neck (Figure 104-10) or cranial abdomen (Figure 104-11) if necessary. Alternatively, partial-thickness osteotomies are performed. Osteotomy is completed with a mallet and osteotome that is positioned parallel to the longitudinal axis of the sternum.
Thoracic Wall
Anatomy
Boundaries of the Thoracic Cavity
Skin
Skeleton
Muscular Anatomy
Nerves and Blood Vessels
Physiology and Pathophysiology
Surgical Approaches to the Thorax
Median Sternotomy
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Wall
Only gold members can continue reading. Log In or Register to continue