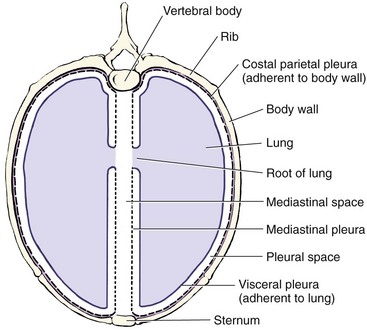
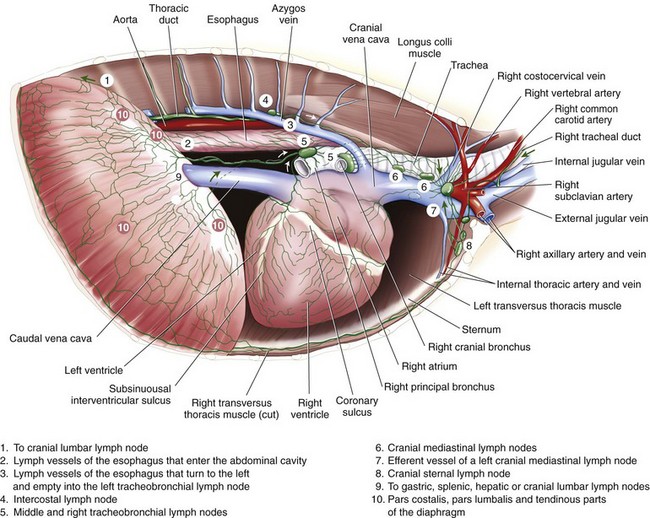
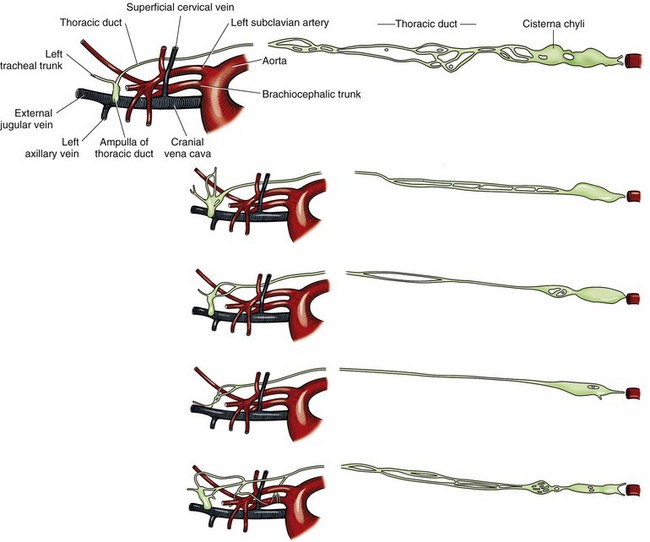
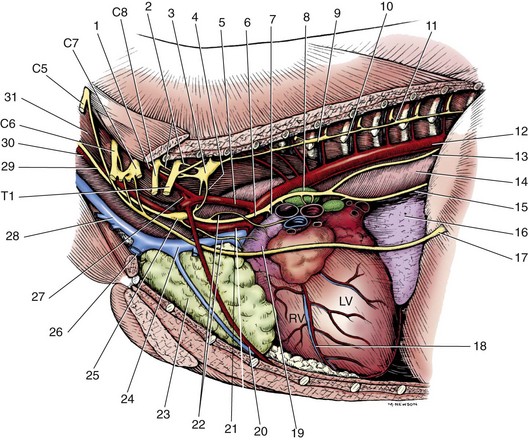
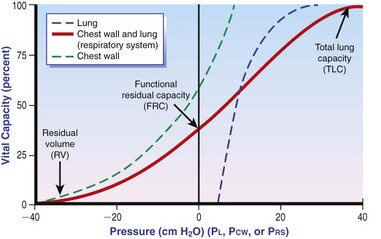
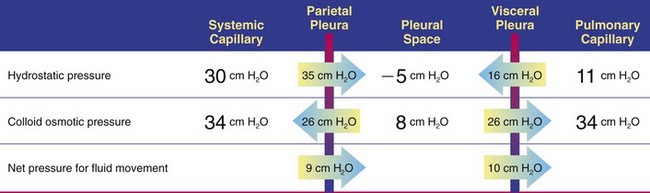
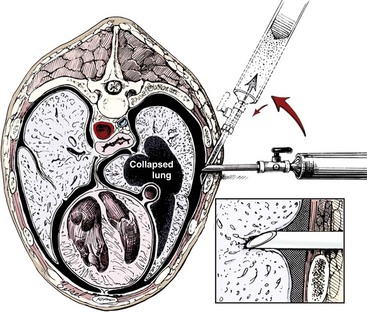
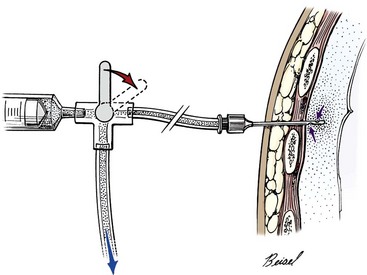
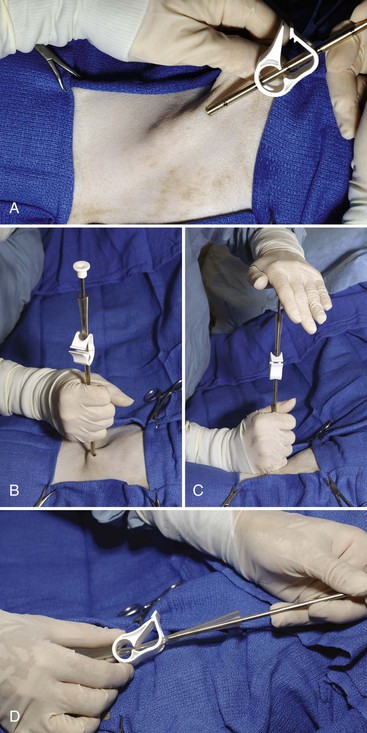
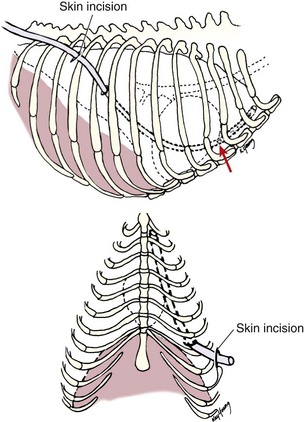
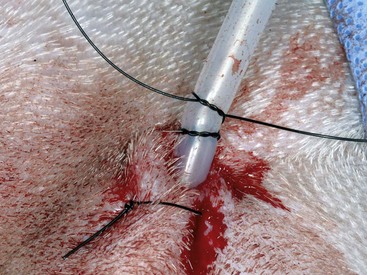
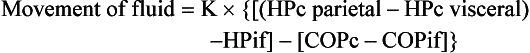Chapter 105 The pleural cavity is a potential space that normally lacks any content except for a film of fluid.28 It exists only as a real cavity when fluid or gas collects between visceral and parietal pleura. The normal pleural space is lined by a single layer of mesothelial cells; these cells are immediately surrounded by elastic connective tissue that contains vascular and lymphatic channels. Stomas between mesothelial cells of the parietal pleura become more numerous ventrally along the thoracic wall and diaphragm, increasing drainage of lymph from the parietal pleura.11 Within the pleural space, fluid is normally small in volume (2 to 3 mL) and low in cell number (<500 cells/µL) and protein content (<1.5 g/dL).30 Pleural fluid lubricates the surfaces of the lungs and surrounding structures to facilitate motion during the ventilatory cycle. The right pleural cavity is larger than the left because of left-sided displacement of the caudal mediastinal wall. The apical portion of each pleural sac extends through the thoracic inlet into the base of the neck, forming pleural-lined pockets known as cupula. The left pleural cupula is larger than the right and extends farther cranially. The anatomy of the diaphragm and thoracic wall is discussed in Chapters 85 and 104. The pleura is usually described as parietal or visceral (pulmonary).28 Visceral pleura tightly adheres to pulmonary surfaces, including the interlobar fissures. Parietal pleura consists of costal, mediastinal, and diaphragmatic portions (Figure 105-1). Costal pleura that lies over the inner surfaces of the ribs is thin and firmly adherent. Over the intercostal muscles, aorta, and related structures, it becomes thicker and less firmly attached. Mediastinal pleura covers the heart and great vessels; trachea; esophagus; thymus gland; internal thoracic vessels; thoracic duct; phrenic, vagal, recurrent laryngeal, cardiosympathetic, and cardiovagal nerves; mediastinal lymph nodes; and longus colli muscle. It contains a delicate, expansive portion ventral to the heart that attaches to the sternum. The ventral mediastinal pleura forms recesses that cradle the ventral borders of the lung lobes. In older dogs, the ventral mediastinal pleura can billow from the thoracic inlet to the level of the diaphragm. Over the heart, mediastinal pleural sheets separate and enclose the fibrous pericardium. It is not clear whether mediastinal pleura completely separates the thoracic space into right and left pleural cavities in dogs and cats. Lymphatic drainage of the thorax includes parietal and visceral groups of nodes (Figure 105-2).28 The visceral nodes include those of the mediastinal and bronchial lymph centers. Cranial mediastinal lymph nodes vary in number and shape and are most commonly associated with the large vessels of the heart that run through the dorsal part of the precardial mediastinum. In dogs, the mediastinal lymph nodes are confined to the precardial mediastinum and heart. Mediastinal lymph nodes drain the muscles of the thorax, neck, and abdomen; peritoneal cavity; scapulae; thoracic and last six cervical vertebrae; ribs; trachea; esophagus; thyroid glands; thymus; mediastinum; costal pleura; heart; aorta; and spinal meninges. They also receive efferent vessels from the intercostal, sternal, middle deep cervical, caudal deep cervical, tracheobronchial, and pulmonary nodes. Mediastinal nodes on the left empty into the thoracic duct, left tracheal trunk, or both and on the right into the right lymphatic duct, right tracheal trunk, or both. The bronchial lymph center includes the pulmonary and tracheobronchial lymph nodes, which lie along the initial part of the bronchi. Pulmonary lymph nodes, which are not present in all dogs, receive lymph from the lungs and drain into tracheobronchial nodes. Besides the lungs and bronchi, tracheobronchial nodes also receive lymph from the thoracic aorta, esophagus, trachea, heart, mediastinum, and diaphragm. Tracheobronchial nodes empty into mediastinal or other tracheobronchial nodes. The parietal group, which includes lymph nodes of the dorsal and ventral thoracic lymph centers, is much smaller and less consistent than the visceral nodes. The ventral thoracic center includes a right and left cranial sternal lymph node or a single median node. Sternal nodes receive drainage from the ribs, sternum, serous membranes, thymus, and adjacent muscles. Efferents from these nodes terminate on the right into the right lymphatic duct and on the left in the thoracic duct. The right lymphatic duct may also receive drainage from the peritoneal cavity via the diaphragm. The dorsal thoracic lymph center is small and inconsistent in the dog and includes the intercostal lymph nodes. The intercostal nodes primarily receive afferent lymphatics from the back, shoulder, chest, and abdominal musculature. Intercostal lymphatics eventually drain into the thoracic duct.9 The thoracic duct is the primary channel for return of lymph from most of the body except for the right thoracic limb, shoulder, and cervical region. It begins in the sublumbar region, or between the diaphragmatic crura, as a continuation of the cisterna chyli. The cisterna chyli is a bipartate, dilated, retroperitoneal lymph channel that lies ventral to the first through fourth lumbar vertebrae along the cranial abdominal aorta. In the caudal thorax, the thoracic duct travels dorsolateral to the aorta on the right in dogs and on the left in cats. It crosses to the left in dogs at the fifth or sixth thoracic vertebra; in most members of both species, it terminates in the left external jugular or jugulosubclavian angle. There are significant anatomic variations in the configuration and number of thoracic duct branches and intercommunications in dogs and cats (Figure 105-3). In many dogs, the cranial portion of the thoracic duct divides into two or more branches, with connecting branches forming a coarse, widespread plexus. Some branches of the plexus may terminate on the azygos vein. The thymus is derived from the third pharyngeal pouch. It is relatively large at birth and continues to grow until 45 months of age, at which time it begins to involute. The organ does not atrophy completely, even in old age, but its lymphoid structure is gradually lost and replaced by fat. The thymus lies cranial to the heart within the ventral aspect of the mediastinum (Figure 105-4) and may be bilobed. Divisions between the lobes are distinct caudally; cranially, the lobes are joined by connective tissue. The left lobe occupies an outpouching of the precardial mediastinal portion of the left pleural sac. The right lobe abuts the cranial surface of the pericardial sac. The dorsal aspect of the thymus lies adjacent to the cranial vena cava, phrenic nerves, and trachea. Active and passive movement of the diaphragm and thoracic wall alter pleural pressure, resulting in changes in pulmonary volume and subsequent gas exchange within the lung. The diaphragm is the major inspiratory muscle. As it contracts, the dome of the diaphragm is pulled caudally, enlarging the thoracic cavity. Additionally, simultaneous contraction of external intercostal muscles results in “bucket handle” movement of the caudal ribs, causing the caudal thoracic wall to move outwardly. Pleural fluid mechanically connects the visceral and parietal pleura; thus, outward movement of the thoracic wall and diaphragm results in negative airway pressure and subsequent lung expansion as long as transthoracic (intrapleural) pressure is enough to overcome airway resistance and inward elastic recoil. Peak inspiratory pleural pressures range from −7 to −14.3 cm H2O (mean, −9.34 cm H2O), drawing air into the airways and to the lungs.25 In normal animals, parietal and visceral pleural stiffness are equivalent and are similar to that of the lung, allowing for transmission of pressure changes in each to the others.34 During normal ventilation, exhalation is primarily passive as a result of inward elastic recoil of the lungs and thoracic wall with diaphragm relaxation. With exhalation, air moves from the pulmonary parenchyma, which is subject to positive intrapleural pressure achieved by elastic recoil, and out the airways to the relatively lower atmospheric pressure. End-expiratory pleural pressure ranges from −3.4 to −6.8 cm H2O with a mean of −5.12 cm H2O.25 The volume of air remaining in the lung at the end of normal exhalation (~45 mL/kg) is known as functional residual capacity (FRC). When the lung volume is decreased below FRC, pulmonary vascular resistance is high because the extraalveolar vessels narrow (Figure 105-5). When the lungs inflate to FRC, resistance decreases because of dilatation of these vessels. Inflation above FRC again increases pulmonary vascular resistance as high tension on the stretched alveolar septa flattens alveolar capillaries. Fluid production in the pleural space is based primarily on the relationship of hydrostatic and colloid osmotic pressure differences between the capillary and lymphatic beds of the parietal and visceral pleura. The Starling law describes the effects of differences in pressure on net filtration. The following equation provides the determinants of pleural fluid dynamics, including vascular permeability:87 Because normal systemic and pulmonary capillary hydrostatic pressures are greater than those of the pleural space, pleural fluid production is favored (Figure 105-6). The gradient across the parietal pleura between the systemic circulation and pleural space is greater than that across the visceral pleura between the pulmonary capillaries and pleural space. The osmotic pressures of the systemic and pulmonary vascular beds are also greater than those of the intrapleural fluid, favoring absorption of fluid across the parietal and visceral pleura. Based on summation of net hydrostatic and osmotic pressure gradients, fluid tends to enter the pleural space from the parietal pleura and be absorbed by the visceral pleural lymphatics and capillaries. However, the parietal pleura contains many stomata through which parietal lymphatics may also absorb fluid.65 Absorption via parietal lymphatics is encouraged by movement of the intercostal and diaphragmatic musculature and lungs. Parietal lymphatics may play an important role in cases of pleural effusion in which pulmonary capillary absorption decreases or is insufficient in the face of high-volume effusion. Significant pleural effusion can result in changes in central and peripheral blood pressure. A volume of 20 mL/kg of pleural effusion can increase central venous pressure in cats in the absence of cardiac or pericardial disease.32 The relationship of volume and central venous pressure is not linear in chronically affected cats. Removal of effusate decreases central venous pressure in cats with normal cardiac function.32 Severe pleural effusion was reported to cause right atrial collapse and signs of cardiac tamponade in the absence of pericardial effusion in one dog; signs resolved with treatment of effusion.54 Chronic air or fluid in the pleural space may also result in changes in pulmonary parenchyma. In one dog with chronic pneumothorax, removal of all the air within the pleural space resulted in respiratory compromise, potentially because of increased FRC and dead space and decreased pulmonary compliance.12 Hypoventilation resolved with reinstillation of some air into the pleural space.12 Classification of pleural fluid is usually the first step in developing the definitive diagnosis of the condition. Pleural fluid is generally classified into three categories based on protein concentration and cell count; cell type and fluid specific gravity may also be considered. Laboratory classification of fluid includes transudate, modified transudate, and exudate with protein content and cell count increasing along the classification scheme. Some variation exists in the literature, but general divisions in protein content, specific gravity, and nucleated cell numbers are listed in Table 105-1. Clinically useful classification of pleural fluid includes pure transudate, serosanguineous, sanguineous (or hemorrhagic), inflammatory, chylous, and neoplastic. Table • 105-1 Serosanguineous effusion is most often concurrently categorized as a modified transudate. With a serosanguineous effusion, the red blood cell (RBC) count, and thus hematocrit, is far less than that of peripheral blood. Many conditions can result in serosanguineous effusion via different pathologic routes. Lung lobe torsion causes venous and lymphatic occlusion in the presence of intact arterial blood flow, resulting in significantly increased hydrostatic pressure and subsequent increase in pleural fluid production. Lymphatic absorption is concurrently decreased. Lung lobe torsion may be primary or secondary.65 In patients with preexisting pleural effusion, suspension of pulmonary parenchyma in the effusate may predispose more mobile lung lobes to torsion.65 Other causes of effusion, such as chylothorax, must therefore be ruled out because pneumolobectomy will not result in resolution of the effusion in those cases. Serosanguineous effusion can also be seen in patients with diaphragmatic hernia and hepatic entrapment. Venous and lymphatic obstruction results in hepatic parenchymal congestion and subsequent fluid leakage. With pericardial effusion, vascular hydrostatic pressure increases with development of cardiac tamponade and right heart failure, leading to increased fluid production. Neoplasia can result in various types of effusion from lymphatic or vascular obstruction or secondary hypoproteinemia or inflammation. Diffuse neoplasia, such as mesothelioma or carcinomatosis, commonly results in serosanguineous effusion. Last, idiopathic pleuritis and pleural effusion have been diagnosed in dogs and cats. Idiopathic pleuritis is most often associated with a modified transudate.48 With sanguineous effusion, packed cell volume of the effusion is similar to that of the peripheral blood; however, because of rapid defibrination, the effusate may not clot. Hemorrhage into the pleural space is commonly associated with trauma, which may be blunt, penetrating, or surgical; coagulopathy; and acute lung lobe torsion. Lung lobe torsion can also be associated with chylous or modified transudative effusion.65 When sanguineous pleural effusion is iatrogenic, the source of hemorrhage depends on the approach and procedures performed. Intercostal, internal thoracic, pulmonary, pericardial, mediastinal, and cardiac vasculature are all possible sources. Intercostal vessels are at risk during lateral thoracotomy, which may also traumatize the internal thoracic vessels if extended ventrally on the thorax. Mediastinal vessels may be increased in size and number with inflammatory conditions of the thorax. Tumors within the chest can erode into vessels or adjacent tissue, leading to significant hemorrhage.42 Common tumors include chemodectoma, which may invade adjacent great vessels, and hemangiosarcoma of the right atrial appendage. Certain tumors (e.g., hemangiosarcoma) or diffuse neoplasia can be associated with disseminated intravascular coagulopathy and hemothorax. Coagulopathies associated with rodenticide ingestion usually respond to vitamin K1 supplementation.61 Life-threatening hemorrhage of any cause requires aggressive stabilization and, potentially, surgical intervention. Treatment of blood loss and atelectasis must both be considered before surgical intervention. Supportive care and stabilization are appropriate and may preclude surgery, allowing for resorption of blood from the pleural space. Autotransfusion may be considered as well because bacterial contamination of hemorrhagic pleural effusion is rare. Neoplastic cells may be present in the effusate, and the long-term prognosis should be weighed when considering autotransfusion in these patients. It is unlikely that neoplastic cells in transfusate will significantly decrease the prognosis in a case of neoplastic-induced hemothrax.11 Chylous effusion is usually grossly apparent because the fluid is milky white or white with a pink hue (Figure 105-7). Chyle is classified as a modified transudate, with protein concentrations between 2.5 and 4 g/dL, cell counts below 7000/µL, and specific gravity below 1.032.30 Initially, lymphocytes predominate in chyle; with time, nondegenerate neutrophils may be more numerous because of intrapleural inflammation coupled with systemic lymphocyte depletion. Definitive diagnosis of chyle is made by comparing serum triglyceride and cholesterol concentrations with those of the effusion. With chyle, triglyceride concentration is higher and cholesterol lower in the effusate compared with serum. Other diagnostic tests include detection of chylomicrons in the fluid, which can be stained with Sudan black, or a positive ether clearance test result. For an ether clearance test, a sample of pleural fluid is placed into two tubes, and both are alkalinized with potassium hydroxide. Ether is added to one tube and tap water in the other; whereas ether will cause clearance of chylous fluid, the sample treated with water remains opaque. Pseudochylous effusion has been described in humans but not dogs or cats. With this condition, the cholesterol concentration is higher, and the triglyceride content lower than that of serum, and chylomicrons are not present. Chylous effusion can result from any condition that increases hydrostatic pressure in the cranial vena cava. Subsequent mechanical or functional obstruction of the lymphaticovenous junction leads to dilatation and leakage of thoracic, and potentially pulmonary, lymphatics.10 Chylothorax can also occur with blunt, iatrogenic, or penetrating trauma to the thoracic duct; however, thoracic duct tears usually heal spontaneously with thoracic drainage.38 Most commonly, the underlying cause of chylothorax is not determined, and the condition is therefore classified as idiopathic. Idiopathic chylothorax is associated with thoracic lymphangiectasia. Inflammatory effusions have an increased white blood cell count compared with a pure transudate. Inflammatory effusions can be modified transudates or exudates, depending on their specific gravity and cell content, and are considered septic effusions if bacteria are detected on culture or cytology. Septic exudate filling the pleural space is referred to as pyothorax or thoracic empyema. Septic effusions are usually associated with a much higher nucleated cell count than nonseptic inflammation (see Table 105-1). Lymphocytes, macrophages, and neutrophils may be present with either type of inflammatory effusion; however, neutrophils become degenerate with septic inflammation. Fluid accumulation secondary to intrathoracic inflammation results from increased fluid production secondary to vasodilation and increased vascular permeability. Additionally, fluid resorption decreases because of increased intrapleural oncotic pressure and, in chronic conditions, pleural thickening. Nonseptic inflammation may be caused by diaphragmatic herniation of abdominal organs, neoplasia, chronic chylothorax or lung lobe torsion, infectious diseases (feline infectious peritonitis), or abdominal conditions such as pancreatitis. Exudative effusion can also occur with idiopathic pleural effusion.48 Inflammatory effusion associated with pancreatitis may be a result of focal inflammation of the diaphragm and release of pancreatic enzymes that cause localized fat necrosis and inflammation.11 Pleural exudate associated with feline infectious peritonitis is high in protein (5 to 12 g/dL) and specific gravity (>1.017), and nucleated cells are usually nondegenerate neutrophils. With chronicity, macrophages, plasma cells, and lymphocytes appear.87 Causes of septic inflammation include penetrating thoracic wall trauma; esophageal trauma or foreign body; tracheal trauma; foreign body inhalation and migration through the small airways or lung; caudal cervical trauma or infection, leading to mediastinitis; repeated thoracocentesis; or cervical or thoracic surgery. Extension from a systemic infection, pneumonia, or rupture of a pulmonary abscess less commonly results in septic pleural exudate. In cats, recent work suggests that parapneumonic spread may be the most common route of infection of the pleural space.4–6 Oropharyngeal flora are most commonly isolated in feline septic pleural effusions, and an increased risk is noted in multicat households, leading to speculation that viral upper respiratory infections may play a role in bacterial pleural infection.4–6 Septic pleural exudates are most often polymicrobial infections, which commonly include anaerobes.103 Fluid should also be evaluated and cultured for Actinomyces spp. and Nocardia spp., which are frequently present in chronic septic exudate.76 Common clinical signs associated with pleural effusion include lethargy, exercise intolerance, inappetence, and weight loss.61 Animals can often compensate for slow accumulation of large effusate volumes and a resultant change in FRC until stressed by activity, increased ambient temperature, or anxiety. Rapid accumulation of a large volume of pleural fluid or air results in acute tachypnea, dyspnea, and collapse. Cyanosis, orthopnea, and outward displacement of the thoracic wall may also occur with large volumes of pneumothorax. Animals with intrapleural disease may develop a cough or reluctance to lie down. Other clinical signs depend on the cause of the thoracic disturbance. Physical examination findings consistent with pleural effusion include a restrictive ventilatory pattern and diminished heart and lung sounds, especially ventrally. Most cases demonstrate dyspnea and tachypnea.61 Animals with severe effusion may stand with their elbows abducted and neck extended. Bronchovesicular sounds may be increased dorsally. On thoracic percussion, the ventral thorax is hyporesonant, and a fluid line may be detected. Cats may exhibit apparent “breath-holding” in which inspiration seems forced and prolonged and exhalation is short and somewhat delayed. Fever may accompany exudative effusions. Depending on the primary disease process, a heart murmur, jugular venous distention, hepatomegaly, ascites, lymphadenopathy, or other findings may be present. Significant dyspnea, cyanosis, and collapse may occur in advanced cases of pleural effusion. Radiography is a sensitive method of diagnosing pleural fluid even when volumes are small: as little as 100 mL of fluid in a dog and 50 mL in a cat can be detected radiographically.87 Loss of detail, cardiac silhouette, or diaphragmatic line; the presence of interlobar fissure lines; and retraction of the lung borders from the chest wall occur with pleural effusion. Horizontal beam radiography allows fluid to move with gravity, and pulmonary parenchyma will rise because of air content. In patients with significant dyspnea, dorsoventral (versus ventrodorsal) and standing lateral radiographs are recommended to avoid worsening of clinical signs or decompensation. If needed, radiographs should be repeated after removal of the majority of the effusion to facilitate evaluation of intrathoracic structures. In medium-size dogs, thoracic radiographs taken before thoracocentesis have been used to estimate the amount of pleural fluid.66 Radiographs should be evaluated for evidence of mass effect and primary pulmonary or cardiac disease. For instance, animals with lung lobe torsions will have increased opacity of the affected lobe, which is also emphysematous in 87%.22,65 Other radiographic signs of lung lobe torsion include irregularity, narrowing, or blunting of the affected bronchus (45% to 75% of dogs); displaced bronchus; mediastinal shift; and dorsal tracheal displacement.22,65 The chance of detection of thoracic cavity abnormalities may be increased by obtaining multiple radiographic views. Caudal mediastinal masses are highlighted in a dorsoventral view because of contrasting adjacent pulmonary parenchyma and magnification.46 A dorsoventral view may also allow better definition of the cranial mediastinum. The left cranial pulmonary cupula is better inflated on the ventrodorsal view, however, which aids in differentiating apical pulmonary and mediastinal mass lesions.46 The ventrodorsal view has also been recommended for evaluation of the cranial and caudal mediastinum, caudal vena cava, and accessory lung lobe.13 Left and right lateral views are recommended in any patient with intrapleural disease to reduce the risk of false-positive and false-negative findings and to help localize the lesion. Orthogonal views should be made if radiographic findings are questionable. Intrapleural contrast studies are rarely required but can be done to aid in the diagnosis of diaphragmatic hernia (see Chapter 85). Positive-contrast esophagoscopy is rarely indicated for the diagnosis of esophageal mass lesions or foreign bodies. Care should be taken to avoid barium in cases of potential esophageal perforation. Lymphangiography may be recommended in patients with chylothorax in which a cause is not detected (see Chylothorax below). Thoracic computed tomography (CT) has been used to detect pulmonary metastasis, mediastinal and thoracic wall mass lesions, undiagnosed pleural effusion, lung lobe torsion, bullae or blebs associated with spontaneous pneumothorax, and pathologic changes associated with foreign bodies.* In one study comparing results of radiographs and CT for diagnosis of noncardiac thoracic disease, a change in the diagnosis was made in 13 of 28 dogs and 3 of 5 cats based on CT results.72 In dogs, thoracic CT is generally considered better than plain radiographs for early detection of pulmonary and lymph node metastasis and therefore should be strongly considered in cases of thoracic neoplasia.36,45,70 Contrast enhancement alone, however, may be related to lymph node inflammation and not metastases.36,45,70 Fine-needle aspiration of lesions can be facilitated with CT guidance.36 In one study of dogs and cats with intrathoracic lesions, CT-guided fine needle aspirates were diagnostic in 65%, and CT-guided biopsies were diagnostic in 83%, with non-neoplastic processes more often nondiagnostic on sampling.109 Pneumothorax is reported in 0% to 27% of animals after CT-guided fine needle aspiration, and hemorrhage is noted in up to 30%; these complications usually require no or minimal intervention.109 Fluid analysis may help distinguish underlying causes based on the classification systems previously mentioned; however, because of overlap in characteristics, misclassification of transudates, modified transudates, and exudates may occur when traditional classification systems are used. In one study of 20 cats with pleural effusion, pleural fluid lactate dehydrogenase (LDHp) concentration and ratio of pleural fluid to serum total protein (TPr) were the most reliable aids for differentiating transudates and exudates.111 The sensitivity, specificity, and accuracy were 100% for differentiating transudates from exudates when a cutoff value of LDHp greater than 226 IU/L was used. The accuracy of TPr was 95% with a cutoff value of greater than 0.56. In dogs, a C-reactive protein concentration greater than 4 µg/mL was 100% sensitive and 94% specific for differentiating transudates and exudates, and concentrations greater than 11 µg/mL were 88% sensitive and 100% specific in distinguishing modified transudate and exudates.71 Transudate could not be differentiated from modified transudate, however. In another study, measurement of vascular endothelial growth factor concentrations was not useful for differentiating malignant from nonmalignant conditions associated with pleural effusion in dogs.19 The patient is placed in sternal recumbency for collection of fluid from the ventral third or fourth to seventh intercostal space. Drainage of intrapleural air can be achieved from the middle of the thorax in a laterally recumbent patient. The thoracic wall should be clipped and aseptically prepared. Thoracocentesis can be performed through a transthoracic needle or catheter (Figures 105-8 and 105-9). An over-the-needle catheter may decrease the risk of pulmonary trauma but may kink, limiting the volume of fluid or air retrieved. Connection of the needle or catheter to a syringe with flexible tubing is strongly advised to prevent movement of the needle during syringe aspiration and to allow the needle to easily move with respirations (see Figure 105-9). A butterfly catheter or needle connected to a syringe via an extension set should suffice. A three-way stopcock is placed at the syringe to permit uninterrupted aspiration and drainage. In patients with pyothorax, a large-gauge, over-the-needle catheter of sufficient length may be fenestrated for removal of large volumes of exudative fluid. Appropriate containers for collection of samples and for disposal of large volumes of fluid should be available. The needle is inserted into the patient with the bevel perpendicular to the thoracic wall and facing the pleural space. To avoid pulmonary trauma, the needle should be directed toward the intrathoracic wall surface at approximately a 45-degree angle with the bevel facing the lung (see Figure 105-8) after the pleura has been penetrated. As fluid or air is withdrawn, the atelectatic lung will reinflate and approximate the thoracic wall. Rubbing of the lung against the needle or catheter should signal significant removal of pleural contents. Pleural fluid or air resulting in hypoventilation should be eliminated by thoracocentesis or by thoracostomy tube placement in cases requiring repeated thoracocentesis. Thoracostomy tube placement is usually performed under general anesthesia, so stabilization should be initiated before tube placement. In unstable animals, wire-guided placement of a fenestrated 14-gauge catheter can be performed under sedation; the catheter may provide pleural drainage for up to 1 week after placement.98 Use of parenteral and local analgesics with an anxiolytic may decrease the overall doses of drugs and inhalant anesthesia required for thoracostomy tube placement. Intercostal nerve blocks may also be used to assist with thoracostomy tube placement and provide analgesia in patients with rib fracture. Anesthetic protocols vary with patient status; when selecting a protocol, the clinician should choose drugs that will allow rapid induction so ventilation can quickly be assisted. A thoracostomy tube should be considered for removal of fluid or air in patients that require repeated thoracocentesis and for monitoring hemorrhage, fluid, or air accumulation after thoracotomy.67 Thoracostomy tubes can also be used to deliver intrapleural local anesthetic after thoracic surgery. Commercially available thoracostomy tubes are flexible yet resistant to collapse. They have multiple fenestrations and are marked longitudinally by a radiopaque line that is disrupted by the fenestrations so that intrapleural location of all holes can be verified on radiographs. Commercial tubes are accompanied by blunt stylets that add stiffness or sharp stylets that protrude beyond the distal end of the tube to facilitate thoracic wall perforation. Additional holes may be added by removing the stylet, bending the tube, and excising a portion of the tube. The portion excised should be less than one third the circumference of the tube to avoid accidental tube breakage and should be through the radiopaque line. The same technique can be used for addition of holes in red rubber catheters if these are used for thoracostomy tubes. The tube should be premeasured to avoid entering the most cranial extent of the mediastinum, which is narrow and can obstruct the fenestrations with application of negative pressure to the tube. If a closed clamp will be used postoperatively, it is preplaced on the tube. The tube is inserted through a skin incision in the dorsal third of the tenth or eleventh intercostal space (Figures 105-10 and 105-11). It is tunneled in the subcutis using the stylet or large hemostatic forceps and inserted into the chest through the seventh or eighth intercostal space in the midthorax. Tunneling offsets the skin and pleural entry sites, providing a “flap valve” effect that limits entry of air into the pleural space along the tube’s surface. After the tube penetrates the pleura, the stylet is aimed at the contralateral elbow and held at a fixed depth while the tube is advanced into the thorax. The tube is compressed with the preplaced clamp or a large hemostatic forceps before the stylet is completely removed, and its end is capped with an appropriate adaptor and stopcock mechanism or attached to continuous suction. The tube should be fixed in place to avoid dislodgement or extrapleural migration of fenestrations. A purse-string skin suture is placed around the base of the tube, tightened to appose the tissues without necrosis, and tied with two square knots, leaving suture ends equal in length. The suture is then secured in a “Chinese fingertrap” pattern that proceeds up the tube: suture ends are crossed on the side of the tube adjacent to the patient and tied on the contralateral side with a surgeon’s throw that slightly compresses the tube (Figure 105-12). The suture crossing on the patient side and contralateral surgeon’s throws should be spaced at least as wide as the tube diameter; at least six crossings and surgeon’s throws should be placed. The completed pattern is secured with two square knots (Figure 105-13). An alternative method for securing the tube is to suture a “butterfly tape” (adhesive tape folded across the tube and adhered back to itself) to the skin; slippage occurs along the tube–tape interface as soon as the tape is wet, however, so this technique is not recommended as a sole means of fixation. Placement of a suture around the tube somewhere along its subcutaneous tunnel has been described but is not necessary if the tunnel is adequately long and is not significantly wider than the thoracostomy tube. In animals with loose or mobile skin (e.g., cats or Shar-Peis), the fingertrap suture can be placed separately from the purse-string suture and secured to the last rib or deep muscles to prevent tube migration with skin movement.
Thoracic Cavity
Anatomy
Pleura
Lymph Nodes
Thoracic Duct
Thymus
Physiology
Fluid Gradients
Pathophysiology
Types of Pleural Effusion

Serosanguineous Effusion
Sanguineous Effusion
Chylous Effusion
Inflammatory Effusion
Clinical Signs of Intrapleural Disease
Diagnostic Imaging
Computed Tomography
Thoracocentesis
Technique
Presurgical Considerations
Thoracostomy Tube Placement
Technique
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Cavity
Only gold members can continue reading. Log In or Register to continue