Therapeutics
3.1 Special considerations when prescribing for rabbits
At the time of writing (2012), there are 37 drugs authorized for use in rabbits in the UK. These include an antibiotic, several endo- and ectoparasiticides, an antiseptic preparation and anaesthetic agents. These comprise drugs in the classes POM-Vs (prescription-only medicine-veterinarian), NFA- VPSs (non-food animal-veterinarian, pharmacist, suitably qualified person), ZFAs (zootechnical food additives) and drugs available under the Small Animal Exemption Scheme (SAESs). There is no pain relief or any drugs for treating anything other than infective and reproductive conditions. Clearly if in clinical practice we were limited to these drugs the ability to treat rabbits would be severely curtailed.
The extensive use of rabbits in toxicity studies has led to an abundance of information on the actions of drugs on rabbit tissue, both in vivo and in vitro, but there is a lack of data on their efficacy in the treatment of clinical disease. For most studies toxic doses (LD50, the dose that will cause 50% of the trial population to die) as well as effective doses (ED50, the dose at which a particular drug achieves its desired effect in 50% of the trial population) are calculated and teratogenic effects may be known, but not actual adverse effects. Young healthy rabbits are used for toxicity studies rather than aged, ill individuals that have not been screened for other diseases. Equally, drugs used in trials may not be used in the same way that they would be indicated for clinical use in rabbits. Therefore, the usage of many preparations in the clinical situation is based on anecdotal evidence from practitioners.
Today’s veterinarians have unparalleled access to sources of information that can lead them to make an informed decision regarding the use of medications on cases in their care. There are now several formularies either dedicated to exotic species (Carpenter, 2012) or providing extensive information on drug use in exotic species (Plumb, 2008; Ramsey, 2011), so peer-reviewed information is easy to access. A rapid Internet search can yield useful results; however, the source of the information must be scrutinized carefully. The best information comes from sites such as the Veterinary Information Network (http://www.vin.com) and WikiVet (http://en.wikivet.net/Veterinary_Education_Online), both of which hold a database of information as well as allowing questions to be posed to both colleagues and recognized experts in their field. Equally, access to literature databases such as PubMed (http://www.ncbi.nlm.nih.gov/pubmed) is freely available and allows practitioners to see the original studies on which drug doses are based. There are, however, many sites that are not peer-reviewed (pet-owner forums are a case in point) where the information is based on experience at best and at worst may be dangerous. Many drug companies hold information about the use of commonly used drugs authorized for domestic species in rabbits. This information may come from drugs trials or from a database of anecdotal use by other practitioners; however, it is worth accessing.
Rabbits differ from other species in many ways and there are some aspects of their physiology that can affect their response to medication.
3.1.1 Digestive physiology
Digestive physiology affects the absorption and metabolism of drugs given orally. Substances can be absorbed from the stomach and small intestine into the portal system to be metabolized in the liver before entering the general circulation. This is known as the ‘first-pass’ effect that can alter the activity of therapeutic agents. The first-pass effect has been demonstrated in rabbits (Huang et al., 1981). Conversely, substances absorbed across the buccal mucosa avoid the portal circulation and hepatic metabolism. It is not always possible to extrapolate dosages and effects of an orally administered drug in a rabbit from another species. There are differences between a carnivore, such as a dog, that swallows food without mastication and whose stomach empties completely and a herbivore, such as a rabbit, that chews the food thoroughly and has a stomach that is never empty. The acid pH of the rabbit’s stomach can affect ionization and absorption of drugs.
Caecotrophy effectively recycles ingested material and prolongs the length of time that substances are retained in the gut. Fluids and small particles are retained in the caecum, whereas substances that bind with large particles will be excreted rapidly. The absorption of certain drugs may be affected by increased retention time in the caecum (Guillot et al., 1988). The dual production of hard and soft faeces in the rabbit is a complex process controlled by the fusus coli, which is highly innervated and vascular. The fusus coli is affected by hormones, such as aldosterone and prostaglandins, as well as the autonomic nervous system. Pharmacological preparations can affect digestive function through their effect on the fusus coli. For example, non-steroidal anti-inflammatory drugs (NSAIDs), such as indomethacin, inhibit endogenous prostaglandin formation and inhibit soft faeces production (Pairet et al., 1986). The ingestion of soft faeces from the anus is triggered by a number of factors including odour, which can be affected by medication. Some drugs, e.g. ampicillin, inhibit normal caecotrophy (Escoula et al., 1981).
3.1.2 Microflora of caecum and digestive tract
The caecum is inhabited by a variety of micro-organisms including protozoa and anaerobic bacteria (see Section 1.3.6). The balance of micro-organisms in the digestive tract, especially in the caecum, is influenced by many factors including antibiotics and other medications. Physiological processes alter caecal pH throughout the day in response to the digestion of food and passage of digesta. Diet affects both the composition of digesta that reaches the caecum and its rate of passage through the gut. Low fibre diets reduce gastrointestinal motility and prolong retention time of digesta in the caecum. Diets high in indigestible fibre promote optimal gastrointestinal motility and a healthy caecal microflora. Therefore rabbits fed on high fibre diets may be less susceptible to disruption of the caecal microflora than those fed on an exclusively cereal diet. Stress or pain increases circulating catecholamines or cortisol, which can have an effect on the balance of gut bacteria. Increased glucocorticoid levels increase coliform counts and alter the aerobic-to-anaerobic bacteria ratio in the gut (Straw, 1988). ‘Dysbiosis’ is the condition that results when the natural flora of the gut are thrown out of balance.
3.1.3 Antibiotic toxicity in rabbits
In rabbits, antibiotics can alter the gut flora with potentially lethal effects, yet rabbits are prone to bacterial infections that require antibiotics to effect a cure. There are many areas of confusion over the use of antibiotics to treat clinical disease in pet rabbits. Their use should be limited to cases where a bacterial infection has been identified.
Some antibiotics have the potential to cause enteritis in rabbits by selectively killing certain bacteria within the gut and allowing pathogenic species to proliferate. Clostridium spp., in particular, can proliferate in the caecum or small intestine and cause rapid death due to the effects of enterotoxins. Clostridium spiroforme is the major pathogen in rabbit enterotoxaemia, although Clostridium difficile and Clostridium perfringens may also be involved on rare occasions (Perkins et al., 1995). A particular strain of C. spiroforme is pathogenic to rabbits and produces an iota toxin. Clostridium perfringens type E also produces iota toxin. Antibodies against either organism will neutralize the other’s iota toxin. This cross-reactivity led people to believe that C. perfringens type E was the pathogen in antibiotic-related diarrhoea in rabbits until C. spiroforme was identified in 1983 (Carman, 1993). For antibiotic-associated enterotoxaemia to develop in rabbits, both the antibiotic and the clostridium need to be present. Clostridium spiroforme is not a normal inhabitant of the rabbit gut flora and normal intestinal ecology of the adult must be disrupted before C. spiroforme will colonize (Carman and Borriello, 1984). Glucose is required as a substrate for iota toxin production (Jenkins, 1997) and it is thought that undigested starch in the hindgut of young rabbits fed on cereal diets may increase susceptibility to enterotoxaemia. In young rabbits intestinal absorption of starch is not as efficient as in adults and residual amounts of carbohydrate may reach the caecum to act as a substrate for bacterial fermentation. Adult rabbits appear to digest starch more efficiently, with only small amounts reaching the caecocolic segment unabsorbed (Blas and Gidenne, 1998). Therefore high carbohydrate diets are less likely to predispose to enterotoxaemia in adults.
In addition to Clostridium spp. there are other pathogenic bacteria that can cause intestinal inflammation, enteritis and diarrhoea. Escherichia coli can produce toxins. In a study of the effects of ampicillin and gentamicin on the bacterial flora of the caecum, a strain of Enterobacter aerogenes predominated in rabbits treated with ampicillin, 40% of which died (Escoula et al., 1981).
Most of the information about antibiotic-associated diarrhoea in rabbits has been gained from experimental studies rather than from the treatment of clinical cases. Healthy rabbits are used for experimental investigations rather than those suffering from disease. Usually young rabbits are used in experimental studies rather than adults, which have a different population of caecal microflora and a varying ability to digest starch. The effect of diet, particularly indigestible fibre, on experimental results is largely overlooked. In some studies, the effects of antibiotic administration is an incidental finding during the treatment of a disease such as osteomyelitis or lung abscesses, in which rabbits are used as experimental models. For example, ampicillin and clindamycin are considered to be high-risk antibiotics that will readily induce diarrhoea. Yet in one study (Norden and Budinsky, 1990), rabbits were given high doses of ampicillin (200 mg/kg) three times daily. Half the rabbits died with diarrhoea and inanition but the other half survived. In another study, Mader et al. (1989) compared cefazolin (5 or 15 mg/kg) and clindamycin (70 mg/kg) in the treatment of osteomyelitis. The antibiotics were given by subcutaneous injection every 6 h for 28 days. Seven out of 20 rabbits in the clindamycin group died, 4 with diarrhoea, whereas all the 24 rabbits in the two cefazolin groups survived and none developed diarrhoea.
Experimental investigations of the effects of antibiotics on gut flora in rabbits have given confusing results. For example, Milhaud et al. (1976) and Escoula et al. (1981) reported a high incidence of antibiotic-associated diarrhoea in rabbits given ampicillin, in contrast to Hara-Kudo et al. (1996), who reported a low incidence of diarrhoea in rabbits injected with ampicillin in comparison with other antibiotics. Differences in the route of administration and dosages may explain some of these discrepancies but, in many cases, the overall picture is still inconsistent.
3.1.3.1 Prevention of antibiotic-associated diarrhoea and enterotoxaemia
The risk of treating individual pet rabbits with antibiotics is difficult to evaluate, as much information is anecdotal. There are documented reports of high mortality rates in commercial rabbits treated either accidentally or deliberately with lincomycin at excessive dosages (Maiers et al., 1984; Thilsted et al., 1981).
The choice of antibiotic and route of administration are important factors in the prevention of antibiotic-associated diarrhoea. Clindamycin, lincomycin and oral ampicillin carry a high risk of inducing diarrhoea, whereas enrofloxacin and trimethoprim combinations are apparently safe, even when they are administered orally over a long period of time. Using high levels of antibiotic increases the risk of enterotoxaemia, especially if they are given orally. Weighing the rabbit and calculating an accurate dose reduces the risk of overdosage.
Oral antibiotics are more likely to induce diarrhoea than those given parenterally. Rabbits are surprisingly easy to inject subcutaneously and most owners can be shown how to give injections themselves. In many cases it is easier to inject a rabbit than to medicate it orally. Care should be taken with topical antibiotic preparations as these can be licked off the coat in sufficient quantities to interfere with gut flora. A companion can ingest topical preparation during mutual grooming.
In ‘high-risk’ situations where enterotoxaemia is likely to develop, cholestyramine can be used prophylactically. Cholestyramine is an ion exchange resin that absorbs enterotoxins. It has been shown to prevent experimental enterotoxaemia associated with clindamycin administration and may also be an effective adjunct to treatment (Flecknell, 1998; Lipman et al., 1992). Probiotics may also be helpful in the prevention and treatment of enteritis (see Table 8.2).
The incidence of enterotoxaemia is greater in intensive situations where the environment is contaminated by clostridial spores. Clostridium spiroforme is more commonly encountered in weanling rabbits than in the individual pet animal. Disinfection of buildings by removing organic matter and applying chemical disinfectants can help to reduce the incidence of disease. A sporicidal agent is needed.
3.1.4 Legislation
In the UK, the Veterinary Medicines Regulations govern the authorization, manufacture, distribution and administration of veterinary medicines (http://www.vmd.defra.gov.uk). These regulations prohibit the administration of veterinary medicinal products (POM-Vs) without the authorization of a veterinary surgeon. Veterinary medicinal products are authorized for use for a specific condition in a particular species of animal. There are exemptions to this rule that can permit the use of alternative products. These exemptions are known collectively as the prescribing cascade.
With the limited amount of authorized drugs available, clearly, many of the drugs prescribed for rabbits are done under the cascade. ‘Off-label’ drug usage may be necessary for a variety of reasons and the responsibility for the use of a medicine in this way lies wholly with the prescribing veterinary surgeon. Because the safeguards ensuring safety and efficacy in place for authorized medications have not been implemented, use of ‘off-label’ medicines can pose a potential risk. Sufficient information should be given to the rabbit’s owner to allow informed consent to the use of the chosen medication; this should include other therapeutic options and use of the cascade to choose the medication suitable for the case. A non-authorized drug should show a clinical advantage compared with the authorized product in the specific circumstances under consideration. All information should be included in the clinical notes, and an ‘off-label’ consent form signed.
The exemptions are different for food-producing animals and those kept as companions. In food-producing animals, to ensure that tissue residue implications have been evaluated, only products authorized for other food-producing animals may be used. In companion animals, products authorized for use in any another species or for a different use in the same species may be used. If there is not a suitable veterinary medicine available, then the use of human preparations is acceptable.
Rabbits are kept as both food-producing and companion animals, so their place in the prescribing cascade is ambiguous. The Veterinary Medicines Directorate takes the view that rabbits should be regarded as food-producing animals
unless the veterinarian in whose care they have been placed can be satisfied that neither the particular animal(s) being treated nor their produce will enter the food chain. Where the veterinarian is satisfied that the animal(s) concerned are being kept solely as pets and will not be used for food production then those particular animals may be regarded as companion animals for the purposes of the prescribing cascade. (Veterinary Medicines Directorate, personal communication).
There are people who keep rabbits both as companions and for meat. Exhibition rabbit breeders especially will kill and eat surplus stock. Rabbit breeders seldom present an animal to a veterinary surgeon for clinical examination. Instead, they treat their rabbits themselves with a variety of home remedies and medications obtained from the Internet. Magazines and textbooks on rabbit care often describe the use of medicines such as antibiotics, parasiticides and motility stimulants that are only available on prescription. As a result, rabbit breeders expect veterinary surgeons to supply these medicines on demand, without consultation, and are unaware of the stringent legislation governing the use of veterinary medicines for rabbits. At the time of writing it is possible for rabbit breeders and owners of pet rabbits to obtain prescription medications illegally over the Internet, often by importation from other countries where the regulations on prescribing are different. These medications may not be what they purport to be and their effects are at best unpredictable if not dangerous, and their use should be strongly discouraged. The prescribing cascade severely limits the choice of medicines available for the treatment of rabbits destined to be eaten as meat.
3.2 Veterinary medicines that are used in rabbits
A formulary of products that are used to treat rabbits is given in Table 3.1.
Table 3.1
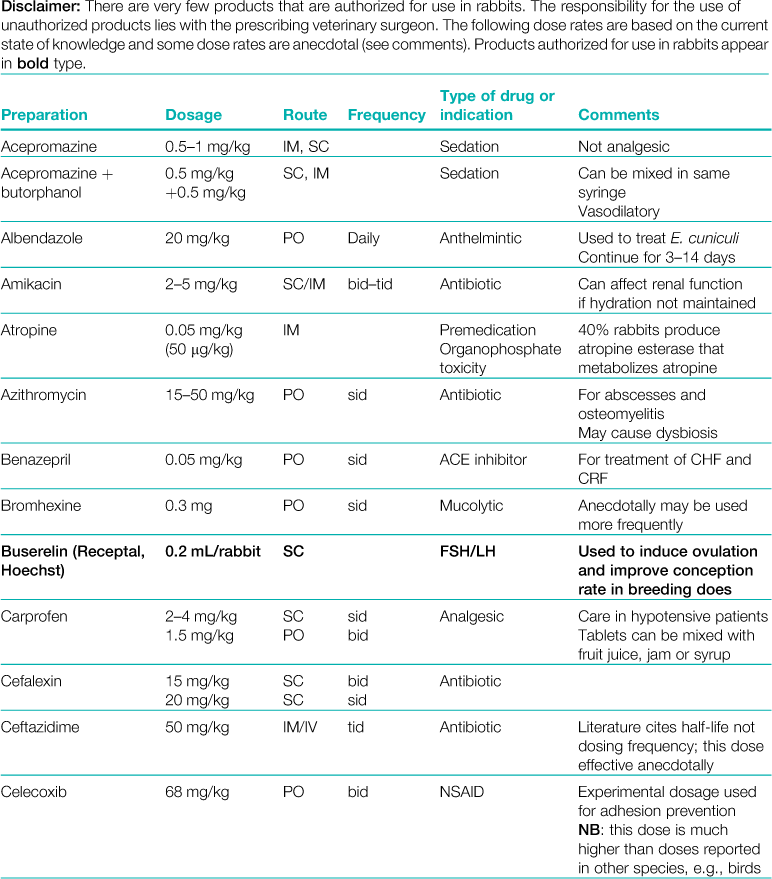
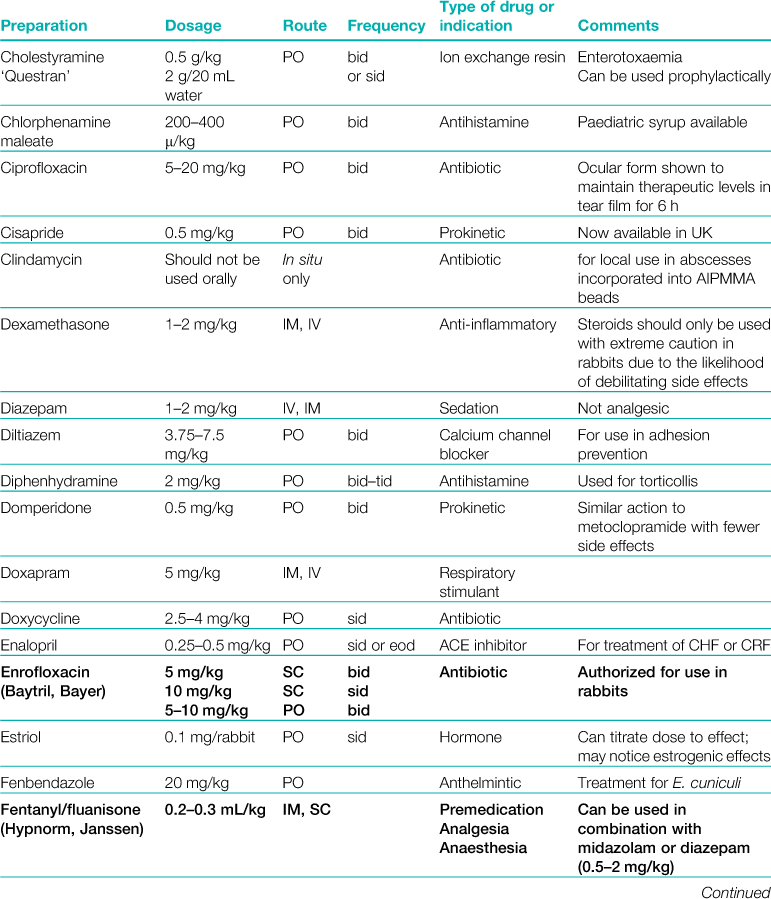
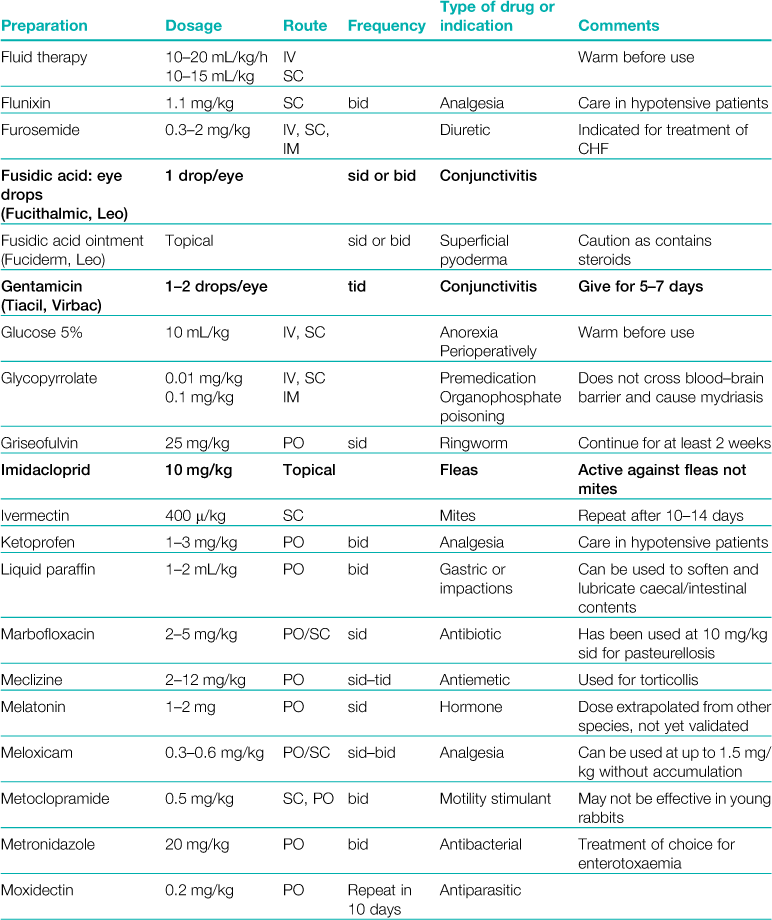
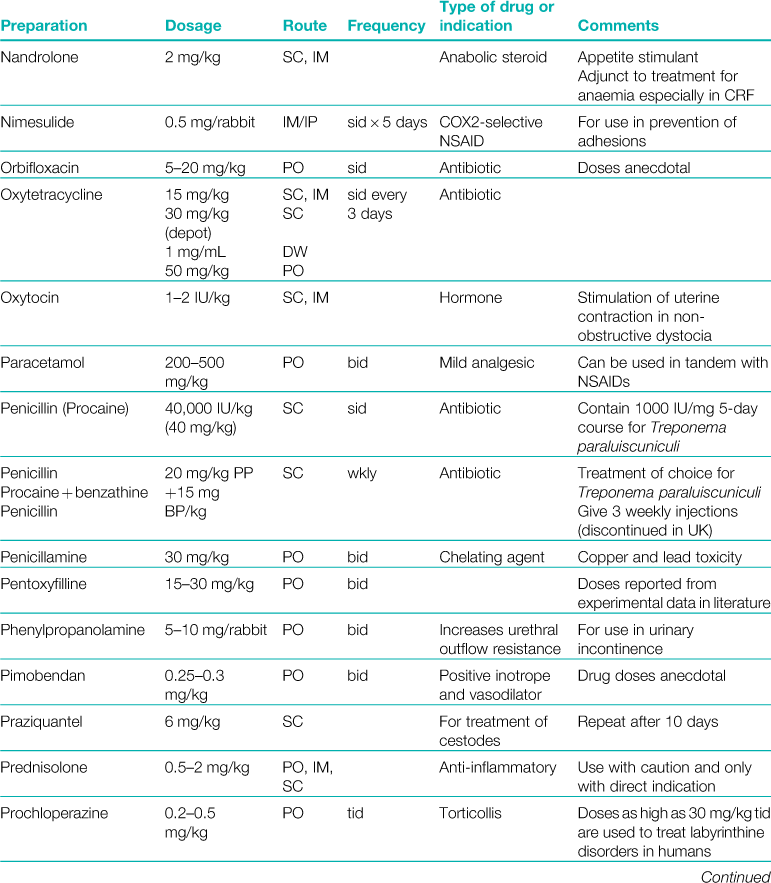
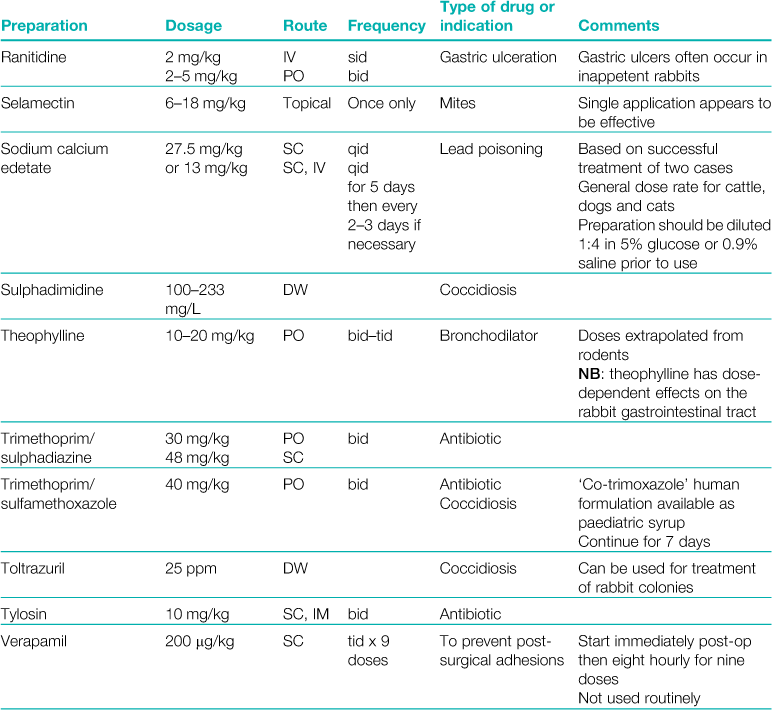
Rabbits drink approximately 10% of their bodyweight daily and eat approximately 5%.
Abbreviations: sid, once daily; bid, twice daily; tid, three times daily; eod, every other day; wkly, once weekly; IV, intravenous injection; SC, subcutaneous injection; IM, intramuscular injection; PO, by mouth; DW, in drinking water; CHF, congestive heart failure; CRF, chronic renal failure.
*Products used during anaesthesia are listed separately in Table 4.1.
3.2.1 Antibiotics
There is a temptation to prescribe antibiotics in any situation where there is an ill rabbit and no specific diagnosis. In view of the risk of antibiotic-associated diarrhoea, it is preferable to reserve antibiotic therapy for situations where there is a definite indication for their use. Antibiotics are most successful for the treatment of primary bacterial infections and for preventing secondary bacterial invasion of tissues damaged by viruses, surgery or other disease. Any antibiotic therapy carries a risk of life-threatening diarrhoea and enterotoxaemia in rabbits. This risk is influenced by diet, choice of antibiotic, dose rate, route of administration, presence of pathogenic clostridial species, age, stress, concurrent corticosteroid therapy and fate. The risk of antibiotic therapy needs to be weighed against the risk of not prescribing antibiotics.
Consideration should be given to the causative pathogen and the efficacy of a particular antibiotic against that organism. The goal of treatment is to achieve an effective concentration of the drug at the site of infection for as long as possible in order to kill the bacteria that is present or likely to be present. Concentrations in excess of the mean inhibitory concentration (MIC) are required. MIC depends on the pharmacokinetics of the antibiotic and the micro-organism at which it is directed. Ideally, culture and sensitivity identify the causal organism and aid antibiotic selection, but this option is not always available in the clinical setting. Infections may be in inaccessible sites such as the tympanic bulla or anterior chamber of the eye. Pasteurella multocida and Staphylococcus spp. are frequently isolated from infected sites. In vitro rabbit isolates of P. multocida are generally sensitive to penicillin, chloramphenicol, tetracycline, erythromycin, novobiocin and nitrofurans with varying susceptibility to streptomycin, kanamycin, neomycin and sulphonamides. They are usually resistant to clindamycin and lincomycin (Manning et al., 1989).
It is important to give therapeutic dosages for an antibiotic to be effective. Medicating the drinking water with antibiotics is unsatisfactory because it is difficult to ensure correct dosages as water intake can vary significantly and the taste of antibiotic can deter the rabbit from drinking the water. Equally, many antibiotics deteriorate in the presence of sunlight. Sweetening the water with sucrose or fruit juices and covering the water bottle can help overcome these problems, but this is still an inaccurate method for providing medication, particularly in pet rabbits.
In a study by Okerman et al. (1990), antibiotics were given to rabbits in the drinking water prior to infecting them with pathogenic P. multocida. In vitro sensitivity of the pathogen was confirmed and trimethoprim sulpha, spiramycin, tetracycline, erythromycin, chloramphenicol and enrofloxacin were tested at typical dose rates. Enrofloxacin was given at three different dosages: 25, 50 and 100 mg/L. Only enrofloxacin at 100 mg/L was effective in preventing infection. Enrofloxacin at 50 mg/L and chloramphenicol prevented rabbits dying but some of the survivors were not in good health. All the rabbits treated with the other antibiotics succumbed to acute pasteurellosis. This study not only highlighted the shortcomings of administering medication in the drinking water but also the importance of giving the correct dose of antibiotic.
It is possible to use potentially toxic antibiotics in a manner that gives high local tissue levels without producing harmful effects. For example, gentamicin, which is known to be nephrotoxic in rabbits, can be injected into abscess capsules or sutured into wounds in the form of antibiotic-impregnated beads with no harmful effect on the kidneys.
3.2.1.1 Ampicillin
Ampicillin and amoxycillin have a similar range of antibacterial activity. There is a range of palatable paediatric syrups containing ampicillin that have been used for rabbits due to their availability and ease of administration. Unfortunately, ampicillin is a ‘high-risk’ antibiotic for rabbits and there are numerous reports of diarrhoea and death following its use. This, in part, is due to the fact that it is excreted well in bile and can potentially be recirculated in caecotrophs. In a study by Milhaud et al. (1976), rabbits were dosed with oral ampicillin at rates of 50, 15 and 5 mg/kg. There was 100% mortality in the 50 mg/kg group and 50% mortality in the other groups. Ampicillin appears to be toxic both parenterally and orally (Escoula et al., 1981). Rehg and Lu (1981) described a fatal case of diarrhoea in a rabbit treated for a respiratory tract infection with 8 mg/kg ampicillin subcutaneously; C. difficile was isolated from the caecum. There are numerous other reports in the literature of the toxicity of ampicillin in rabbits. It has been used to induce experimental C. difficile infection (Guandalini et al., 1988). Ampicillin has no advantages over other antibiotics in the treatment of diseases that affect pet rabbits.
3.2.1.2 Cephalosporins
Cephalosporins are a group of bactericidal, non-toxic antibacterials which contain the β-lactam ring and are closely related to penicillin. On this basis they have been included by many authors in the list of antibiotics not suitable for use in rabbits. Actual reports of antibiotic-associated diarrhoea are scarce if the antibiotic is administered parenterally. In one study, ceftriaxone was administered by daily intramuscular injection for 4 weeks to a group of rabbits without adverse effects (Evans and Nelson, 1993). Cephalosporins are active against a range of Gram-positive and -negative organisms, including Pasteurella spp. (Bishop, 1998) and staphylococcal resistance is less common to cephalosporins than to penicillin.
3.2.1.2.1 Cefalexin
As a cephalosporin, cefalexin has the reputation as an unsafe antibiotic for rabbits (Laval, 1990; Morris, 2000). Reports of adverse effects can be traced back to a German reference in which oral, not parenteral, cefalexin was administered at high doses (Schröder et al., 1982). At standard dose rates of 15–30 mg/kg daily, parenteral cefalexin is well tolerated by rabbits.
Cefalexin appears promptly in the aqueous humour of the non-inflamed eye of rabbits, in concentrations of 15–20% of serum levels (Gager et al., 1969). Cefalexin is resistant to the action of staphylococcal penicillinase and is therefore active against penicillin-resistant strains of Staphylococcus aureus and against P. multocida. It is a useful antibiotic for the treatment of many conditions in pet rabbits, including osteomyelitis, due to good penetration to soft tissues and bone (Harcourt-Brown, 1997). Parenteral cefalexin can be combined with topical cephalonium eye ointment for the treatment of ocular disease. It is an effective treatment for eye infections, especially if there is evidence of uveitis or keratitis. Cefalexin is effective in suppressing signs associated with respiratory infections (Bishop, 1998).
3.2.1.2.2 Ceftazidime
Ceftazidime (Fortum, GlaxoSmithKline) is a third-generation cephalosporin with high activity against many Gram-negative organisms but relatively less activity against Gram-positive bacteria when compared with first- and second-generation cephalosporins. Because of its good activity against Pseudomonas, its use should be limited to cases where sensitivity to its action is confirmed or where infections are acute or severe. Anecdotally it has been used safely in rabbits, although due to the lack of pharmacodynamics and pharmacokinetic data the doses are empirical.
3.2.1.3 Lincosamides (clindamycin and lincomycin)
Lincomycin is not recommended in rabbits, and has been associated with enterotoxaemia.
3.2.1.3.1 Clindamycin
This antibiotic can induce fatal antibiotic-associated diarrhoea if administered orally. There is evidence that it is safer if given parenterally (Lucore et al., 1986), but there is no parenteral preparation available for use in the UK although there is an injectable formulation in the USA. Many of the strains of P. multocida that affect rabbits are resistant to clindamycin (Manning et al., 1989). Other bacteria such as Staphylococcus spp., which can be isolated from abscess cavities, may be sensitive to clindamycin. Local administration of clindamycin into abscess cavities has been described (Chappell, 1994). An antibiotic capsule is punctured and placed in the cavity after surgical drainage and debridement. The skin is sutured to retain the capsule. This simple, cheap technique removes the necessity of administering antibiotic by other routes. However, consideration must be given to the possibility of the antibiotic either being absorbed from a thin-walled abscess or reaching the oral cavity through a draining fistula. Antibiotic-associated diarrhoea, which is usually fatal, can result from a clindamycin capsule placed in a site that is groomed or licked by the rabbit or its companion.
3.2.1.4 Fluoroquinolones
3.2.1.4.1 Enrofloxacin
Enrofloxacin (Baytril, Bayer) is a fluoroquinolone that is active against a wide range of Gram-negative and some Gram-positive micro-organisms. It is active against Pseudomonas spp. and Mycoplasma spp. At the present time (2013), enrofloxacin is the only systemic antibiotic authorized for use in rabbits. Pasteurella multocida is very sensitive to enrofloxacin in vitro (Mähler et al., 1995). Enrofloxacin is indicated for the treatment of bacterial infections of the alimentary and respiratory tract. After administration by the oral or subcutaneous route, the drug is rapidly distributed through the tissues before being eliminated. In order to maintain minimum inhibitory concentrations of enrofloxacin for P. multocida, 12-hourly dosing of 5 mg/kg is required either orally or parenterally (Broome et al., 1991). Unfortunately, this regimen may not achieve sufficient tissue concentrations to eliminate infection from the nasal cavity, trachea, middle ear and outer ear where P. multocida frequently resides (Mähler et al., 1995). In a study by Okerman et al. (1990), oral administration of enrofloxacin via the drinking water was effective against experimental challenge with highly pathogenic P. multocida infection. A dose rate of 10 mg/kg was found to be more effective than 5 mg/kg. In a study by Suckow et al. (1996), experimental P. multocida intranasal infections of pregnant does were not eliminated with enrofloxacin given either in the drinking water (200 mg/L) or intramuscularly (5 mg/kg bid), although the antibiotic prevented transmission of the organism to the kits. There is no documentary evidence that enrofloxacin disrupts intestinal flora or predisposes to enteric problems even when administered orally. It is a very safe antibiotic in rabbits and can be given over long periods. There is evidence that quinolones can cause arthropathy in juvenile rabbits (Sharpnack et al., 1994).
3.2.1.4.2 Marbofloxacin
Marbofloxacin (Marbocyl, Vetoquinol) is a newer fluoroquinolone that has a broad spectrum of activity against many bacteria and mycoplasmas. It is bactericidal and its effect is concentration dependent, which allows pulse dosing regimens to be effective (Rougier et al., 2006). Ideally its use should be restricted to those infections where there is confirmed susceptibility and other antibiotics are unlikely to be effective. While cartilage abnormalities have not been proven to be caused by marbofloxacin, the same precautions observed with enrofloxacin should be employed and its use in growing animals avoided until further information is available.
3.2.1.4.3 Orbifloxacin
This is a new-generation fluoroquinolone (Orbax, Santa Cruz Biotech), with an activity profile similar to that of marbofloxacin. Similar qualifications should be applied to its use and, at the time of writing, while it is likely to be safe and effective in rabbits, there is little information on its use in this species.
3.2.1.5 Fusidic acid
Fusidic acid is a steroidal antibiotic isolated from the fermentation products of the fungus Fusidium coccineum. It is chemically related to cephalosporin P1 (Taylor et al., 1987) and has bacteriostatic or bactericidal activity mainly against Gram-positive bacteria by selectively inhibiting bacterial protein synthesis (Bishop, 1998). The antibiotic is available for veterinary use as topical preparations. It is particularly effective against pathogenic staphylococci (Saijonmaa-Koulumies et al., 1998). Fusidic acid can penetrate avascular tissue even in large collections of pus (Taylor et al., 1987). Topical application penetrates the cornea and aqueous humour of rabbits, giving levels of fusidic acid well above minimum inhibitory concentrations for most Gram-negative organisms for at least an hour after application. Minimum inhibitory concentrations persist for up to 24 h in the cornea against Gram-positive infections (Taylor et al., 1987). Fusidic acid viscous eye drops (Fucithalmic Vet, Leo Laboratories) give significantly higher tear fluid concentrations than chloramphenicol viscous eye drops (van Bijsterveld et al., 1987) and the preparation has sustained release properties. The carbomer base increases the concentration of fusidic acid in the tear film so this preparation is useful for the treatment of conjunctivitis and keratitis in rabbits, especially as it only needs to be applied once or twice daily. The gel preparation of fusidic acid in combination with betamethasone (Fuciderm, Leo Laboratories) or without betamethasone (Fucidin, Leo Laboratories) can be used to treat inflamed perineal skin folds. The ointment is applied once daily for 2–3 days after the area has been clipped and cleansed and the underlying problem addressed. Fusidic acid does not appear to cause antibiotic-associated diarrhoea. Steroid preparations should be used with caution in rabbits.
3.2.1.6 Aminoglycosides
3.2.1.6.1 Gentamicin
Gentamicin is an aminoglycoside that is bactericidal and active against Gram-negative organisms and some Gram-positive ones, but not streptococci. Antibiotic resistance by enteric organisms can occur rapidly, especially if subtherapeutic doses are given (Bishop, 1998). Absorption does not occur from the digestive tract, so oral administration is ineffective unless enteric infections are being treated. The antibiotic is ineffective against anaerobes and is not indicated for enteric infections in rabbits. Gentamicin does not appear to cause disturbances in caecal microflora (Escoula et al., 1981). It is poorly distributed in the eye, brain or cerebrospinal fluid. Excretion is solely by the kidney and the drug is potentially nephrotoxic. Purulent material binds and inactivates aminoglycosides (Elliott, 1998), so gentamicin is only effective for the topical treatment of abscesses if all the necrotic material has been removed by thorough debridement.
Parenteral gentamicin was not found to be as effective as penicillin in the treatment of rhinitis in a group of laboratory rabbits infected with P. multocida (Gaertner, 1991). As gentamicin is potentially nephrotoxic, its systemic use is not recommended.
The main indications for gentamicin in rabbits are for topical treatment of conjunctivitis and for local treatment of abscesses. Gentamicin ophthalmic solution (Tiacil, Virbac) is one of the few authorized preparations available for rabbits. Gentamicin is poorly absorbed into the inflamed eye and not at all in the normal eye of rabbits (Behrens-Baumann, 1996).
Gentamicin is also used in rabbits for the treatment of abscesses. It can be packed into abscess cavities incorporated into beads of polymethylmethacrylate (bone cement). The technique of implanting antibiotic-impregnated substances into infected wounds or compound fractures has increased in human medicine in recent years and gentamicin is often used. It has been routinely incorporated in the bone cement used in human hip replacements. Gentamicin withstands the exothermic process that takes place during curing of the cement. In veterinary medicine, septic arthritis in both horses and cattle has been successfully treated using gentamicin-impregnated polymethylmethacrylate beads (Butson et al., 1996). The beads can be purchased ready-made as 7-mm spheres (Septopal, Merck) or made in a variety of sizes from gentamicin-impregnated bone cement (see Section 6.3.4). Gentamicin (Pangram, Bimeda UK) can also be injected into the wall of abscesses (Brown, 1998).
3.2.1.7 Metronidazole
Metronidazole (Flagyl, Winthrop) is a nitroimidazole that is bactericidal to most Gram-negative and many anaerobic Gram-positive bacteria. It has negligible activity against aerobic Gram-positive infections and is not effective in the treatment of pasteurellosis. Nitroimidazoles have antiprotozoal properties, although in vitro testing suggests that metronidazole is ineffective against Encephalitozoon cuniculi (Franssen et al., 1995). Metronidazole has been cited as a treatment of choice for enterotoxaemia caused by C. spiroforme (Carman, 1994) and has been found to be effective in preventing abscess formation after experimental septic peritonitis in rabbits (Simopoulos et al., 1994). The antibiotic is safe and easy to administer. Paediatric suspensions are available for oral dosing in addition to veterinary preparations suitable for parenteral administration.
3.2.1.8 Penicillin
Procaine penicillin can be used on its own or combined with benzathine penicillin as a depot injection. Penicillin is generally active against staphylococci, β-haemolytic streptococci and Pasteurella spp., although many staphylococci are now becoming resistant to the antibiotic. It is inactive against Bordetella bronchiseptica. In rabbits, penicillin is the treatment of choice for venereal spirochaetosis caused by Treponema paraluiscuniculi.
Penicillin is an example of conflicting information about the safety of a particular antibiotic in rabbits. Penicillin is often cited as a high-risk antibiotic for causing enterotoxaemia (Laval, 1990) but it has been used extensively to treat both venereal spirochaetosis (Cunliffe-Beamer and Fox, 1981) and pasteurellosis (Gaertner, 1991; Jaslow et al., 1981) without complications. Benzylpenicillin is particularly active against Gram-positive aerobes and anaerobes including Clostridium and Bacteroides spp. (Bishop, 1998). Clinically, it seems that penicillin is a safe antibiotic to use parenterally but not orally. There are no indications for its oral use.
Penicillin is useful for the treatment of pasteurellosis. In order to achieve blood levels that reach minimum inhibitory concentrations against P. multocida, penicillin needs to be given 8 hourly by intramuscular injection (Welch et al., 1987), although daily injections of long-acting preparations have been used to overcome the practical problems associated with 8-hourly injections (Gaertner, 1991).
Many reference sources give dosages of penicillin in international units (IU). There are 1000 IU per milligram.
Long-acting depot injections are useful for rabbits. A combination of short-acting procaine penicillin is combined with benzathine penicillin that is slowly absorbed and maintains therapeutic blood levels over 3–4 days. Toxic effects from the procaine component have been described if high doses are used (Harkness and Wagner, 1995). There are reports of the death of kits when nursing does have received procaine penicillin. The deaths have been attributed to the toxic effects of procaine (Collins, 1995).
3.2.1.9 Potentiated sulphonamides
Sulphonamides are an antibacterial group of compounds that act by competing with tissue factors such as p-aminobenzoic acid. They have a wide range of activity. They are effective against some protozoa, including Toxoplasma and coccidia (Bishop, 1998). Sulphonamides diffuse well into body tissues. They are partly inactivated in the liver. The acetylated derivatives are relatively insoluble in acid urine and can precipitate in renal tubules, leading to crystalluria and renal failure. Rabbits excrete alkaline urine and are therefore less likely to develop crystalluria and subsequent renal damage as a result of sulphonamide therapy. However, kidney function is a consideration when selecting any therapeutic agent, including sulphonamides and good hydration should be maintained.
Sulphonamides can be combined with folate reductase inhibitors such as baquiloprim, ormetoprim or trimethoprim to form a preparation that is bactericidal and has a wide antibacterial spectrum including anaerobic bacteria. The half-life of some sulphonamides that are combined with trimethoprim is shorter in rabbits than in other species. For example, sulphadiazine, which is the sulphonamide component of most veterinary preparations, only has a half-life of about an hour in rabbits compared to 5–10 h in other species (Morris, 2000).
Potentiated sulphonamides diffuse well into body tissues and are a good choice of antibiotic for rabbits because of their low toxicity, availability and ease of administration. However, potentiated sulphonamides are inactivated by exudate and debris (Whittem and Gaon, 1998) and are not as effective in the treatment of established purulent infection. There are no reports of antibiotic-associated diarrhoea with potentiated sulphonamides and so trimethoprim combinations can be used safely as a prophylactic antibiotic during surgery where wound contamination could occur.
Sulphonamides are used to treat coccidiosis in a number of species including rabbits. They have activity against the spectrum of life cycle stages. In most cases, they are only available in large quantities suitable for medicating large numbers of farmed rabbits. For the individual rabbit, co-trimoxazole is a trimethoprim/sulphamethoxazole combination that is available for human use. A paediatric oral suspension (Septrin, Aspen) that can be used to treat coccidiosis is available. Alternatively, sulphadimethoxine is available for treatment of coccidiosis in pigeons and may be purchased in small quantities.
Oral dosing is straightforward using either paediatric syrups or preparations suitable for piglets. Prolonged administration of certain sulphonamides can cause keratoconjunctivitis sicca in dogs and antagonize vitamin K in poultry.
3.2.1.10 Tetracyclines
Tetracyclines are broad-spectrum antibiotics that are effective against Mycoplasma and Chlamydia spp. and some protozoa as well as a range of Gram-positive and -negative bacteria. Tetracyclines are bacteriostatic and many organisms, especially S. aureus, are now resistant to their effects. Tetracyclines have a synergistic effect with tylosin against Pasteurella. Many rabbit isolates of P. multocida are susceptible to tetracyclines in vitro. In the past, tetracyclines have been used extensively in commercial rabbit units both as growth promoters and prophylactically. They were administered either in the feed or in drinking water. However, it has been demonstrated that the administration of tetracycline in the drinking water does not produce detectable levels of the antibiotic in the serum, even at a high dosage (1600 mg/L). Medication of the water is also associated with a significant drop in water intake (Percy and Black, 1988). Therefore, this method of administration is unlikely to be effective. High parenteral dosages (30 mg/kg every 8 h) resulted in anorexia and diarrhoea in a study by McElroy et al. (1987), so this antibiotic does have toxic potential in rabbits, although the risk is small. It is safer than many other antibiotics. The main indication for tetracyclines is in the treatment of Tyzzer’s disease caused by Clostridium piliforme (Harkness and Wagner, 1995). Depot injections of oxytetracycline or doxycycline have been used for the prolonged treatment of pasteurellosis or abscesses in pet rabbits (Laval, 1990; Malley, 1995).
3.2.1.11 Macrolide antibiotics
3.2.1.11.1 Tylosin
Tylosin is a macrolide antibiotic with good activity against mycoplasmas and Gram-positive organisms (Bishop, 1998). It is bacteriostatic. There are reports of its use in rabbits, although its efficacy or potential for inducing enterotoxaemia is unclear and its use is not currently recommended. There are safer and more well-evaluated drugs available to cover most of the spectrum of activity of this drug.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


