Urogenital Diseases
12.1 Anatomy of the urogenital tract
The anatomy of the urogenital tract is illustrated by Barone et al. (1973). The rabbit kidney is unipapillate with extensive evaginations of the pelvis into the medullary tissue. The rabbit is the only known mammal in which the tubules can be separated from kidney slices with the tubular epithelium intact. Rabbit kidneys are therefore used for many in vitro studies of renal function. The right kidney can be palpated in the thoracolumbar region and is cranial to the left kidney. There may be substantial amounts of perirenal fat in some individuals, which displaces the kidneys ventrally. Both kidneys are usually visible on abdominal X-ray plates (see Box 8.1). The thin-walled bladder occupies the ventrocaudal abdomen.
In the female the urethra opens into a vestibulum in the vagina called the urogenital sinus (Cruise and Brewer, 1994). A bulbourethral gland is situated in the dorsal wall of the urogenital sinus and the clitoris lies along the ventral surface. The external margins of the urogenital sinus form the vulva. The vaginal body is a flaccid structure which can retain urine. The uterus is bicornuate with a cervix on each horn.
The sexually mature male has two external testicles that lie on either side of the penis in two sparsely haired scrotal sacs. The testicles descend at approximately 10–12 weeks. During periods of food deprivation or illness, the testicles can be withdrawn into the inguinal canal by the well-developed external cremaster muscle. The vas deferens enters a seminal vesicle that opens into the prostatic segment of the urethra. A vesicular gland lies with the prostate gland in the dorsal wall of the seminal vesicle. A small pair of bulbourethral glands forms a bilobed swelling in the dorsal wall of the urethra immediately posterior to the prostate (Cruise and Brewer, 1994).
Externally, immediately adjacent to the penis or the vulva, lie two deep inguinal spaces which are closely associated with inguinal glands that secrete a thick brown or white waxy exudate into the space (see Figure 1.11). Immediately dorsal to the genital opening is the anus.
12.2 Renal function in rabbits
The function of the rabbit kidney differs from that of more familiar species in some clinically significant ways. Rabbits are very susceptible to the effects of acid–base disturbances, pain, stress, anorexia and dehydration because of their renal responses. The role of the rabbit kidney in calcium homeostasis results in the excretion of large amounts of calcium that form a calcium carbonate precipitate in the alkaline urine, giving it a turbid appearance in healthy animals (Table 12.1).
12.2.1 Renal response to acid–base disturbances
Rabbits excrete alkaline urine and have a limited ability to excrete hydrogen ions (see Section 1.3.12). As a result, they are susceptible to acid–base disturbances. Some of the renal compensatory mechanisms present in other species are absent from rabbits. Carbonic anhydrase, an enzyme which catalyses the reversible conversion of carbon dioxide to bicarbonate, is present in tubule epithelial cells in large amounts in species such as humans, monkeys and rats, in comparison with the rabbit (Dobyan et al., 1982).
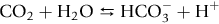
Some workers believe that the relative lack of carbonic anhydrase in the renal tubular epithelium is compensated for by the presence of the enzyme in the red blood cells, leaving no significant difference in hydrogen-transporting capabilities under normal conditions (Effros and Nioka, 1983).
In other mammals, ammonia is produced in the kidney by glutamine deamination in response to a fall in plasma pH, or a decreased concentration of bicarbonate. It is another mechanism for dealing with acidosis by removing H+ ions in the form NH4+. In rabbits, glutamine deamination only takes place in response to reduced serum bicarbonate concentrations but not a drop in plasma pH. It can be stimulated by an infusion of phosphate ions (Yu et al., 1976). The alternative biochemical pathways that are present in other species and result in ammonia synthesis appear to be absent in the rabbit (Brewer and Cruise, 1994). As a result of their limited ability to excrete hydrogen ions, rabbits are susceptible to metabolic acidosis. Rabbits are not as sensitive to the effects of loop diuretics as other species due to the insensitivity of the inner medullary collecting duct cells (Brewer and Cruise, 1994).
12.2.2 Stress and renal blood flow
In rabbits, pain and stress have a significant effect on renal blood flow. Experimental studies suggest that adrenaline is responsible for a marked and prolonged reduction in renal plasma flow and glomerular filtration (Brod and Sirota, 1949). In a study by Kaplan and Smith (1935) into the effects of diuresis and urine flow in rabbits, the forcible oral administration of large amounts of water (> 40 mL/kg every 30 min) was fatal in some rabbits. They became oliguric, convulsed and died. The experiment was repeated using a single dose of 50 mL/kg of water before subjecting the rabbits to unpleasant or painful stimuli. In all cases the disturbing stimuli were immediately followed by a marked decrease in urine flow, renal plasma flow and filtration rate. Oliguria was frequently severe, lasting from 30 to 120 min. Some rabbits that were infused with water at 0.3 mL/min during this period died in convulsions. Rabbits that were not stimulated and remained undisturbed could withstand diuresis by increasing urine flow.
In a later study of the renal circulation in rabbits (Korner, 1963), the author found that it was ‘particularly important to keep the animals in their experimental cages without restraint, to avoid handling the animals when collecting blood and urine samples, to avoid overhydration by the administration of excessive water loads and to prevent dehydration by the prolonged use of strong osmotic agents’. These conclusions are relevant to the handling and treatment of the pet rabbit, especially with regard to fluid therapy.
12.2.3 Calcium excretion
Calcium metabolism in rabbits is described in Section 1.3.12. Briefly, calcium is readily absorbed from the intestine in rabbits (Cheeke and Amberg, 1973). Calcium absorption from a diet with adequate calcium concentrations appears to be passive and vitamin D independent (Bourdeau et al., 1986; Kamphues et al., 1986). Blood calcium concentrations are higher and more variable in rabbits than in other species. Calcium homeostasis is mainly regulated by the kidney, which responds rapidly to changes in calcium status. Responses are mediated by PTH and 1,25-dihydroxyvitamin D3 (Bourdeau et al., 1988) and result in excretion rates of calcium that are proportional to dietary intake (Kennedy, 1965). The kidney plays a vital role in calcium regulation in rabbits. During periods of calcium deprivation, tubular reabsorption of calcium by the kidney increases (Bourdeau and Lau, 1992). During periods of high calcium intake, the kidney can increase the excretion of calcium into the urine considerably (Whiting and Quamme, 1984). Urinary calcium excretion is also increased during periods of restricted phosphate intake (Bourdeau et al., 1990; Depalo et al., 1988). Calcium carbonate is formed in the alkaline urine of rabbits and forms a white precipitate.
12.3 Urine examination
There are a variety of ways in which urine can be collected from rabbits (see Section 1.10.3.3). Many rabbits will urinate in an empty litter tray or urine can be collected by cystocentesis. Normal rabbit urine varies in its visual appearance. The colour can vary from the pale yellow colour through a range of oranges and browns to a deep red colour that can be mistaken for blood. The colour depends on the diet and variations are the result of the excretion of plant pigments. Vegetables such as beetroot, cabbage, broccoli and dandelions often result in the excretion of red urine. There are also some clinical conditions, such as urolithiasis or uterine disorders, that can cause red urine due to haematuria. Examination of the urine with a dipstick (Hemostix, Ames) will often differentiate between blood and plant pigments; however, if the urine is concentrated and strongly coloured this may affect reading the stick. Alternatively, the urine may be examined microscopically for the presence of red blood cells, or a Wood’s lamp can be used as urinary pigments fluoresce when exposed to ultraviolet light (Benson and Paul-Murphy, 1999). Normal rabbit urine is turbid due to the presence of calcium carbonate precipitates. The amount of precipitate varies with the calcium content of the diet and the health, age, and reproductive and hydration status of the rabbit. Individuals with high calcium requirements such as young rabbits and pregnant or lactating does usually produce clear urine. High calcium diets, dehydration and urine retention can result in large amounts of calcium carbonate precipitate that forms a thick sludge in the bladder and causes urethral irritation and dysfunction. It is sometimes difficult to differentiate between normal calcium carbonate deposits and abnormal amounts of sludge. Normal rabbit urine can be radiopaque on abdominal radiographs.
In addition to the presence of calcium carbonate crystals, ammonium magnesium phosphate crystals are also found in normal rabbit urine. The specific gravity of urine can be difficult to evaluate accurately due to the presence of mineral deposits (Goad et al., 1989) but is approximately 1.003–1.036. The urine is naturally alkaline, with a pH of 8–8.2. Traces of glucose and protein can be present. Urine can be spun and the sediment examined microscopically for the presence of crystals, red cells, inflammatory cells and bacteria. Urine cultures can confirm bacterial infection and aid antibiotic selection.
Haematuria may be caused by blood from the reproductive or urinary tract. The list of differential diagnoses is similar to other species (see Table 1.7). In entire female rabbits, uterine disease is often present. Uterine adenocarcinomas, polyps or endometrial venous aneurysms can rupture and bleed intermittently. In rabbits, blood from the uterus is often voided mixed with urine because the vaginal vestibule fills with urine during micturition. Blood clots may be present in the urine in association with uterine disease. Urolithiasis and/or cystitis can also cause haematuria. Chronic polypoid cystitis, renal infarcts and disseminated intravascular coagulopathy have all been described as causes of haematuria in laboratory rabbits (Garibaldi et al., 1987).
12.4 Lower urinary tract disease
Like cats, pet rabbits are prone to a variety of interacting urinary tract disorders that can be grouped together. Feline lower urinary tract disease (FLUTD) or feline urological syndrome (FUS) is also called ‘the fat lazy cat syndrome’ (Blood and Studdert, 1999). An analogy can be made with the fat lazy pet rabbit, which is also prone to lower urinary tract disorders. The exact aetiology is unknown but there appear to be many predisposing factors (see Table 12.2). In rabbits, the syndrome includes urinary incontinence, ‘sludgy urine’ and cystic calculi. Ureteral calculi and nephrolithiasis are also seen and can cause eventual renal failure.
Clinical signs of lower urinary tract disease in rabbits include inappropriate urination, depression, a hunched posture, teeth grinding, dysuria, perineal scalding, urinary incontinence, polyuria and polydipsia (Table 12.2). Visual examination of the urine, urinalysis, sediment examination and culture can be used to establish the presence of cystitis and bacterial infection. Examination of the perineum and consideration of the patient’s mobility, husbandry and general state of health are important as there is an inter-relationship between the predisposing causes. Abdominal radiography is nearly always indicated for evaluating the spine, kidneys, ureters, uterus and bladder. Further investigations such as examination of the oral cavity for the presence of molar spurs, or serological testing for E. cuniculi, may be required. Ultrasound examination can also be helpful, especially if uterine disease is suspected. Evaluation of renal function is necessary in rabbits with urolithiasis, especially if there are stones in the kidney or ureters.
12.4.1 The role of hypercalcaemia and hypercalciuria in urinary tract disease in rabbits
The susceptibility of pet rabbits to urinary tract disorders is often attributed to excessive dietary intake of calcium. It is postulated that high dietary calcium results in hypercalcaemia, hypercalciuria and the accumulation of calcium deposits in the urine. This association between the rabbit’s unusual calcium metabolism and the development of urinary tract disorders warrants further investigation as there are other factors, apart from high dietary calcium levels, that predispose to urinary tract disorders. The rabbit’s kidney is adapted to the excretion of calcium and the presence of sediment in the urine is a normal finding in many rabbits. In a study of the haematological and biochemical parameters of pet rabbits with dental disease, a comparison was made with a group of free-range rabbits with access to natural vegetation. Total serum calcium concentrations as high as 4.28 mmol/L were recorded in the free-range group, who showed no evidence of urinary tract disease other than turbid urine, which was considered to be normal (Harcourt-Brown and Baker, 2001). The free-range group lived out their natural life without evidence of any urinary tract disorders.
Although high dietary calcium intake is not always associated with urinary tract disease, a low calcium intake does appear to prevent the development of sludgy urine and related disorders. The incidence of urinary tract disease was much higher in the USA than in the UK. Historically, most pet rabbits were kept in hutches and fed on mixed cereal rations in the UK. Selective feeding from mixed rations can result in a calcium-deficient diet (Harcourt-Brown, 1996). Calcium deficiency and/or vitamin D deficiency results in metabolic bone disease that predisposes to dental disease (see Section 5.5.1.1). With the advent of several brands of high-quality monocomponent diets formulated for rabbits in the UK, calcium deficiency has become less common in the pet rabbit population. This has led to a parallel increase in the incidence of lower urinary tract disease in this country. This is not to suggest that pelleted diets are responsible, as lack of mobility, longer life span and varying social factors have all played their part. In recent years monocomponent diets that are low in calcium have been produced in recognition of this condition, in an attempt to try to counteract the problem. While there has been an increase in lower urinary tract disease within the population, the levels of dental disease have remained relatively consistent.
Dietary phosphorus levels are an important factor in the urinary excretion of calcium. Experimentally, dietary phosphorus restriction results in substantial hypercalciuria (Bourdeau et al., 1990; DePalo et al., 1988). Calcium and phosphorus are mobilized from bone in response to hypophosphataemia and excess calcium is excreted in the urine. Phosphorus is deficient in the soil of some parts of the world and hay and mature herbage are poor sources of the mineral in comparison with cereals. The availability of phosphorus in alfalfa is low in rabbits (Cheeke et al., 1985).
Urine retention in association with large amounts of calcium deposits in the urine can result in urinary tract disease. There are many behavioural and physical reasons for pet rabbits to retain urine. Wild rabbits urinate frequently. They do not urinate in their burrow, but do so above ground over landmarks or other rabbits’ terrain as part of their territory-marking behaviour. Neutered rabbits do not void urine and territory mark as much as their entire counterparts. The stimulus to urinate and mark territory is absent from solitary rabbits with no neighbours to threaten their territory. Sedentary pet rabbits are often forced to urinate in a hutch and may retain urine for as long as possible. Rabbits that are overweight or suffer from painful conditions such as spondylitis or sore hocks are reluctant or unable to adopt the correct position to urinate (see Figure 12.1) and can retain urine as a result. Urine retention in rabbits leads to the sedimentation of the urine within the bladder. During urination, the supernatant is voided and the sediment is retained in the bladder. Eventually the sediment forms a thick, viscid sludge-like toothpaste (see Figure 12.2). Secondary bacterial infection and urinary incontinence ensue.

Figure 12.1 Normal urination stance.
Rabbits that are given the freedom to exercise and mark their territory urinate frequently. In order to direct the jet of urine away from the body, the rabbit lifts its hindquarters and raises its tail. Failure to adopt the correct stance for urination can result in urine contaminating the fur around the genitalia and scalding the skin.
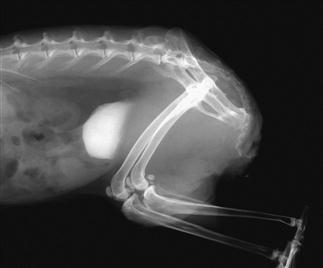
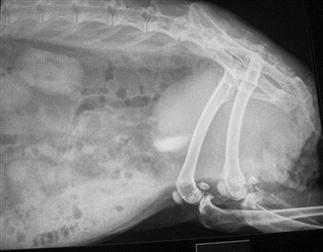
Figure 12.2 Lateral view of the caudal abdomen of a rabbit with ‘sludgy urine’.
A lateral view of the caudal abdomen of a 3-year-old neutered male dwarf lop house rabbit is shown. There is a quantity of radiodense sediment in the bladder. The rabbit was suffering from urine scalding of the perineum and inner thighs (see Figure 12.4). He was maintained on a diet of hay and vegetables with a small amount of complete extruded rabbit food each day. Total serum calcium concentration was 3.17 mmol/L, which is within the reference range for laboratory rabbits. Ionized blood calcium was 1.67 mmol/L. The bladder was enlarged and tense. Palpation of the bladder evoked straining that would produce a small amount of urine. The bladder after the rabbit had urinated is shown. The urine that remained within the bladder had formed a thick sediment that was impossible for the rabbit to void. Under general anaesthesia, the bladder was emptied by gentle, manual compression. The urine that was expressed was thick and viscid (see Figure 12.5). Radiography also revealed a displaced 7th lumbar vertebra and a narrowed lumbosacral disc space. The rabbit could not adopt the correct position to urinate. He was euthanized. Sludgy urine is discussed in Section 12.4.2.
12.4.2 ‘Sludgy urine’
Calcium carbonate deposits that build up in the bladder result in the accumulation of a thick paste or sludge (Figure 12.3). The sludge is irritant to the bladder lining, cystitis develops and blood can be present in the urine. Affected rabbits are depressed and adopt a hunched position. Urination appears to be painful; the sludge in the urine causes urethritis, worsening urine retention. The bladder feels enlarged and turgid. Palpation of the bladders appears to be uncomfortable for the rabbit and often evokes a straining response. Urine is passed in small quantities and may dribble from the urethra. Perineal scalding occurs (see Figure 12.4). Voided urine may appear only slightly turbid but radiographically the bladder is filled with radiodense sediment (see Figure 12.2). Under general anaesthesia, copious quantities of a viscid sludgy material can be expressed from the bladder (see Figure 12.5). There appears to be a difference in the urine from rabbits with ‘sludge’ and normal urine containing calcium carbonate deposits. The sediment in sludgy urine forms a dense precipitate, whereas calcium carbonate deposits in normal urine can easily be shaken up to form a suspension. Sludgy urine is distressing for the rabbit. The sludge irritates the bladder, urethra and perineal skin, causing pain and irritation. Secondary infection and cystitis is common. Superficial bacterial dermatitis, pain and a reluctance to urinate exacerbate the condition. Urethritis leads to urinary incontinence.
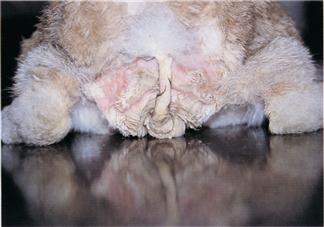
Figure 12.4 Urine scalding.
Any condition that prevents a rabbit from adopting the correct stance for urination can result in urine retention or urine scalding of the perineal skin. Once the skin is inflamed, urethritis makes urination painful and a vicious circle begins (see Figure 12.3). The rabbit illustrated is a 3-year-old neutered male dwarf lop house rabbit that was suffering from urine scalding of the perineum and inner thighs. He had urine retention and sludgy urine (see Figure 12.2). Radiography revealed a displaced 7th lumbar vertebra and a narrowed lumbosacral disc space. As a result of the spinal lesion, the rabbit could not adopt the correct position to urinate. Figure 12.5 shows the urine from this rabbit.

Figure 12.5 A comparison of normal urine with sludgy urine.
There are differences in the way in which sediment forms in sludgy and normal urine. The two samples on the left of the picture are sludgy urine that was expressed from the rabbit illustrated in Figures 12.4 and 12.2. The far left sample was the urine that was initially expressed from the bladder. The middle sample was thick viscid urine that had apparently been retained in the bladder for some time. It had the consistency of toothpaste. A fine sediment that set like concrete formed in both these samples after they were left standing for a few hours. The sediment could only be broken up by shaking the sample vigorously. The sample on the right is urine from a normal rabbit that also contains sediment. However, the sediment in the normal sample easily forms a suspension with gentle shaking, even after it is left undisturbed for 48 h or more. Analysis of both samples is similar. They both contain calcium carbonate and some calcium oxalate crystals, which give urine its radiodense appearance on radiographs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree