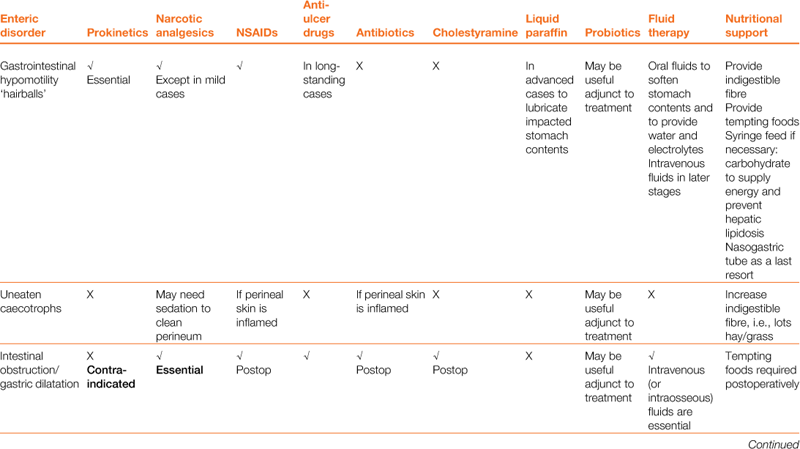
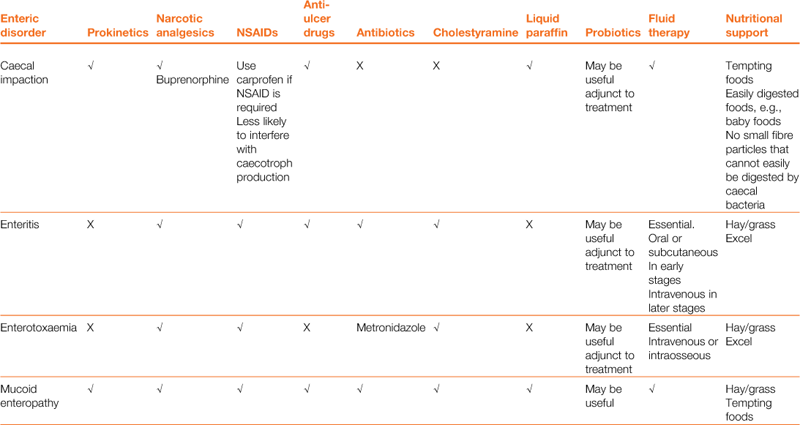
Digestive Disorders
A wide array of digestive disorders affect rabbits. Diarrhoea, bloat, scours, mucoid enteritis, enterotoxaemia, gut stasis, wool-block, trichobezoars, enteritis, gastroenteropathy and mucoid enteropathy are among the variety of non-specific terms used to describe the diseases of the gastrointestinal tract. Many of the digestive disorders that afflict pet rabbits are related to diet and only a few are caused by enteric pathogens. There is a complex inter-relationship between the predisposing factors and causes of digestive disease. This inter-relationship is summarized in Figure 8.1. Enteric disease is manifested by a disruption in normal faecal production. The consistency and frequency of hard and/or soft faeces are altered. There may be mucus production. The term ‘diarrhoea’ can be confusing, both to owners and to vets, and it is necessary to discriminate between true diarrhoea and a failure to eat caecotrophs. A list of differential diagnoses of ‘diarrhoea’ is given in Table 8.1.
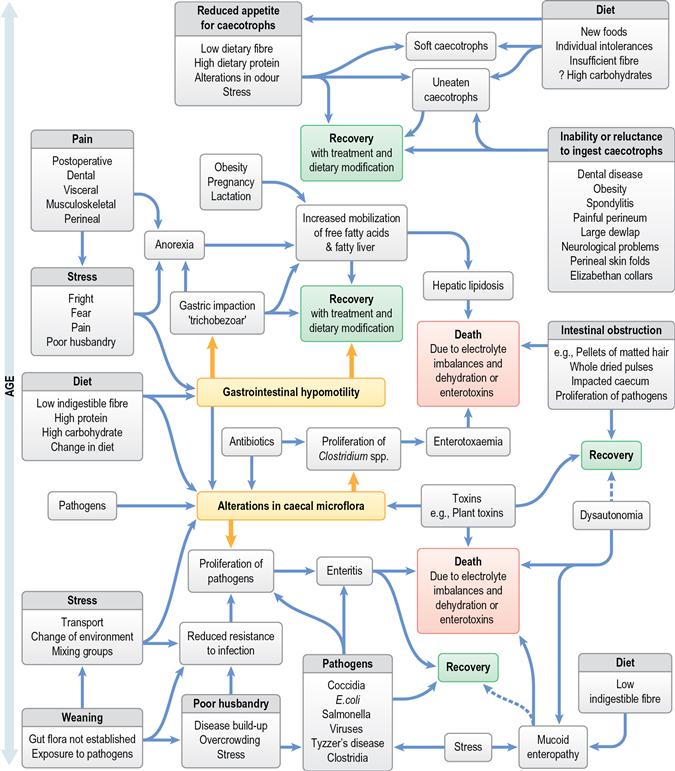
Figure 8.1 Inter-relationship of predisposing factors and causes of gastrointestinal disease in rabbits.
Table 8.1
Differential diagnosis of ‘diarrhoea’ in rabbits
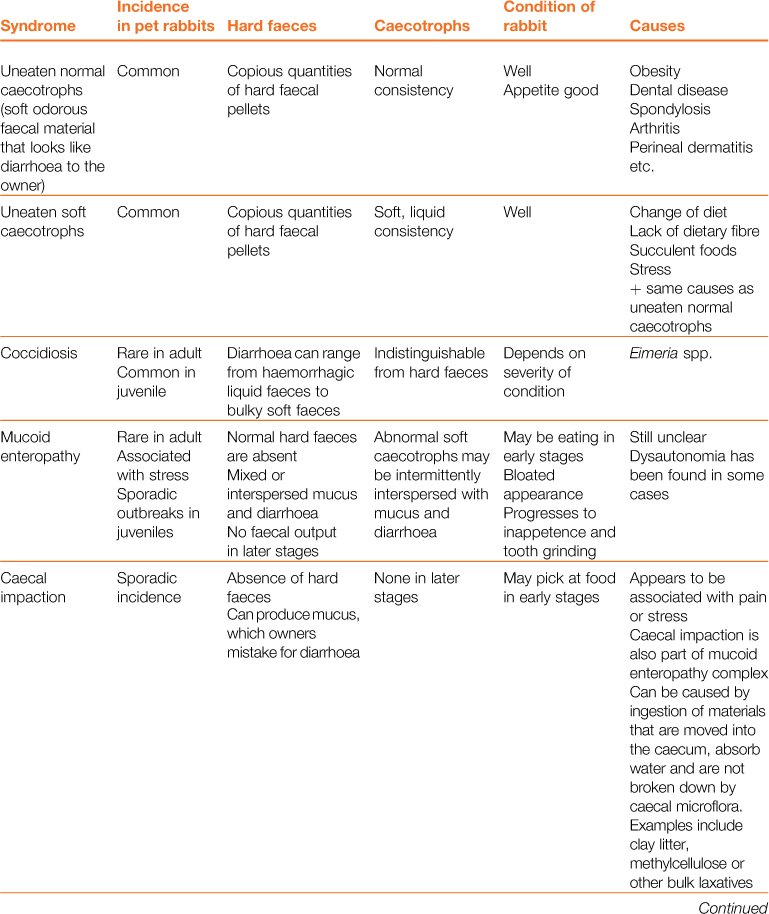
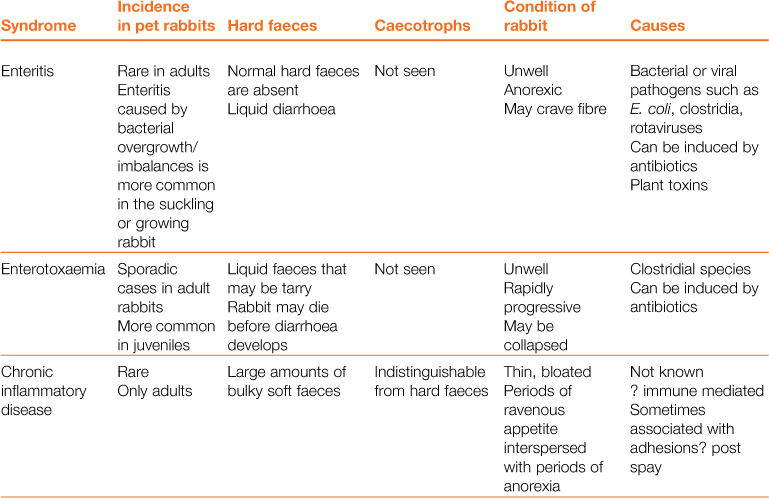
In other species, diarrhoea is manifested by the frequent evacuation of watery droppings (Blood and Studdert, 1999). Rabbits produce two types of droppings, i.e. hard faeces and caecotrophs. Normal caecotrophs are soft in consistency and are often mistaken for diarrhoea. The nature and frequency of both types of faeces are an important consideration. In some conditions, there is also excessive mucus production that can be mistaken for diarrhoea.
8.1 Digestive physiology
A detailed description of the rabbit’s digestive physiology is given in Section 1.3.1. Briefly, the rabbit is a strict herbivore whose digestive system is adapted for the ingestion of a fibrous diet. Digestion in the stomach and small intestine is similar to monogastric animals and food that reaches the hindgut is mainly composed of fibre that cannot be broken down by the digestive enzymes of the stomach and small intestine. The rabbit has the ability to separate large fibre particles from small fibre particles in the proximal colon. The small particles and the large undigested particles are simultaneously sent in opposite directions. Large particles of undigested fibre pass distally and are excreted in hard faecal pellets. Small fibre particles are sent in a retrograde direction into the caecum where they undergo bacterial fermentation. Bacterial fermentation within the caecum releases volatile fatty acids that are absorbed as an energy source. The result of bacterial fermentation within the caecum is a fine paste containing amino acids, vitamins, enzymes, micro-organisms and volatile fatty acids.
Therefore, the rabbit’s colon has a dual function. For most of the day, it mixes and separates ingesta, simultaneously sending indigestible particles towards the anus, and fermentable particles towards the caecum. Periodically, the motility of the proximal colon alters completely, and pasty caecal contents are directed along the colon to be expelled as soft faecal pellets or caecotrophs. Caecotroph production follows a diurnal rhythm. Most rabbits produce soft faeces during the morning and evening approximately 4 h after feeding. These caecotrophs are re-ingested directly from the anus to be digested in the stomach and small intestine as an additional source of nutrients for the rabbit. The nature of the intestinal contents, muscular activity, transit time and exchange of water and electrolytes alter according to the type of faeces passing through the colon. Therefore, digestion and colonic motility can be in either the ‘hard faeces phase’ or the ‘soft faeces phase’ (see Figure 1.4). The amount of ingesta and gas in the various sections of the digestive tract alters according to the phase of excretion. The size and shape of the caecum also follows a diurnal rhythm, which is an important consideration during abdominal palpation or radiography of rabbits.
Separation of ingesta in the proximal colon is accomplished by a combination of functional anatomy and colonic motility. Sacculations in the wall of the proximal colon (haustra) retain small particles while the larger particles accumulate in the lumen. Haustral activity directs small particles towards the caecum, while segmental activity directs large particles towards the anus. Lagomorphs have a specially adapted muscular segment of the colon known as the fusus coli that contains a large number of mucus glands (see Figure 1.5). The fusus coli acts as pacemaker for colonic motility. It is highly innervated and vascular and it is not only controlled by the autonomic nervous system but also subject to the effects of metabolites and hormones such as aldosterone and prostaglandins. During the excretion of caecotrophs, haustral activity ceases and caecal material is moved swiftly along the large colon. In the fusus coli, the material is formed into pellets that become encapsulated in mucus (see Figure 1.5). The transit time for soft faeces through the colon is 1.5–2.5 times faster than that for hard faeces (Fioramonti and Ruckesbusch, 1976).
Within the caecum lies a complex ecosystem of microflora nourished by water and digesta that arrive from the small intestine via the proximal colon. Water is secreted into the proximal colon during the process of mixing and separating and is sent into the caecum with the small particles. Water is absorbed from the caecum across the caecal wall into the circulation. The retention time of digesta within the caecum is affected by both caecocolonic motility and the nature of ingesta that reaches it. Conditions within the caecum are affected by the type and amount of nutrients that supply the microflora and the products of bacterial fermentation. The balance of micro-organisms in the caecum is of paramount importance to the health of the animal. A healthy microflora digests food efficiently. Any factor that upsets the balance of caecal microflora has the potential to result in the proliferation of pathogenic bacteria and cause disease.
8.2 Inter-relating factors in digestive disease
8.2.1 Intestinal microflora
The caecum is a finely balanced ecosystem composed of a variety of micro-organisms nourished by a constant supply of water and nutrients from the small intestine. Changes in the amount and content of the ingesta that reaches the caecum have an effect on the balance of micro-organisms, which are therefore dependent on diet and intestinal motility. Bacteroides spp. predominate in a microflora composed of aerobic and anaerobic Gram-positive and -negative rods, cocci, filaments, coccobacilli and spirochaetes. In addition to the aerobic flora, over 74 strains of anaerobic bacteria have been isolated from the caecal mucosa and many of these species have not been cultivated (Straw, 1988). The microbial flora can contain small numbers of potential pathogens such as Clostridium spp. Stress has an effect on caecal microflora. Increased glucocorticoid levels increase coliform counts and narrow the aerobic-to-anaerobic bacteria ratio in the gut (Straw, 1988). Changes in the caecal microflora can be seen in Gram-stained smears of caecal contents. In the healthy rabbit, high numbers of large anaerobic metachromatic bacteria (LAMB) and protozoa are present. In rabbits suffering from mucoid enteropathy, a drop in the number of LAMB and protozoa and an increase in coliforms are found (Lelkes and Chang, 1987).
The caecal microflora synthesize volatile fatty acids that are absorbed across the caecal wall into the circulation. Anaerobic Bacteroides are the principal source of butyrate that is used as an energy source for the caecal epithelium. The caecal epithelium is adapted for the efficient absorption of water and electrolytes. Butyrate is also important in the regulation of caecal pH, which has an optimum of 5.7–6.1. Changes in caecal pH alter the caecal microflora and can result in the proliferation of pathogens. The proportions of volatile fatty acids in the caecum influence appetite and gut motility. In healthy rabbits, acetates predominate, followed by butyrates and propionates. Low fibre diets result in decreased acetates and increased propionates and butyrates. An increase in caecal butyrate inhibits normal peristalsis of the gut (Lang, 1981).
The microflora of the rabbit’s digestive tract changes according to the age and diet of the animal. In wild rabbits and those pet rabbits that eat a natural diet of grass and hay, a healthy gut flora that is resilient to any minor dietary changes that occur as the result of eating novel foods becomes established. In contrast, in intensive situations where large numbers of rabbits are kept in a small space and fed on artificial diets containing products that rabbits would not normally eat, alterations in the intestinal microflora can rapidly result in the proliferation of pathogens and the development of enteritis. Large numbers of pathogenic bacteria are most likely to be present in intensive situations. Commercial rabbits are young, growing animals, in which a healthy caecal microflora has not become established. Because of the financial importance of losses due to enteric disease in commercial units, extensive research has been carried out into the effects of varying dietary protein, carbohydrate and fibre levels on caecal microflora and volatile fatty acid production. These considerations are beyond the remit of this book, which is mainly concerned with the individual pet rabbit and not commercial rabbit production. The nutrition of the commercial rabbit is described in detail in P. R. Cheeke’s Rabbit Feeding and Nutrition (Academic Press, Orlando, 1987) and reviewed in The Nutrition of the Rabbit (C. De Blas and J. Wiseman, eds) (CAB Publishing, Wallingford, Oxford, 1998).
8.2.2 Diet
It is not possible to consider any digestive problem in rabbits without examining the diet. The role of fibre and its ‘digestibility’ or ‘indigestibility’ is an important concept in the understanding of digestive disease in rabbits. Fibre digestion depends upon the presence of cellulolytic bacteria within the digestive tract. In rabbits the term ‘digestibility’ includes bacterial degradation or fermentation within the caecum, and not just digestion in the stomach and small intestine. For this reason, the term ‘fermentable fibre’ may be less confusing than ‘digestible fibre’. Bacterial fermentation of fibre within the caecum varies according to the chemical structure and particle size (see Figure 1.6). For example, hemicellulose is more digestible than lignin. Small particles enter the caecum where their digestibility is affected by their size. Smaller particles of fermentable fibre have a relatively greater surface area for bacteria to adhere to and are digested more quickly than the larger particles. Very large particles (> 0.5 mm) do not enter the caecum and are expelled, undigested, in the hard faeces. Although it has no nutritive value, indigestible fibre is an essential part of the diet because it stimulates gut motility, which sends nutrients and fluids into the caecum for bacterial fermentation. Insufficient indigestible fibre results in slow gut motility and retention of hair and ingesta in the stomach. Reduced motility in the proximal colon affects the separation of intestinal contents and reduces the supply of digesta and fluid to the caecum. Lack of both fermentable and indigestible fibre results in changes in caecal pH, volatile fatty acid distribution and the balance of microbial flora. Alterations in the populations of micro-organisms can allow enteric pathogens to proliferate. Therefore, although a low level of dietary fibre may not actually cause disease, it is a major predisposing factor. Additional factors such as coccidiosis or treatment with ampicillin are needed to cause pathological disease in commercial rabbits fed on a low fibre diet (Licois and Mongin, 1980). The caecal appendix is larger in rabbits on a low fibre diet, suggesting either an increased demand for the buffering effects of bicarbonate or an increase in lymphoid activity in response to greater production of bacterial toxins (Cheeke et al., 1986).
The amount of indigestible (non-fermentable) fibre in the diet has an effect on appetite, both for food and for caecotrophs. High levels of indigestible fibre promote caecotroph ingestion, whereas high protein levels reduce it. More food is consumed by rabbits on a diet high in indigestible fibre, i.e. large particles of lignified plant material. However, processing the food to reduce the indigestible fibre down to particles small enough to enter the caecum will suppress appetite. Lignin is not digested by the caecal bacteria, whereas cellulose, hemicellulose and pectin are. Excessive amounts of ground lignified material in the caecum increase retention time and decrease digestibility (Chiou et al., 1998). Caecal impaction can be the result.
8.2.3 Age and husbandry considerations
The digestive disorders of the young rabbit, especially around the time of weaning, are very different from the adult pet. The stomach pH of suckling rabbits is approximately 5–6.5. Adult rabbits have a stomach pH of 1–2, except during digestion of caecotrophs, when the pH rises. The high pH of suckling rabbits not only permits healthy bacteria to pass through the digestive tract and colonize the hindgut but also permits the passage of pathogens, such as pathogenic strains of Escherichia coli. Susceptibility to pathogenic strains of E. coli varies with age; rabbits of 3 weeks are more susceptible than rabbits of 6 weeks of age (Licois et al., 1992). Weaning is a stressful period for rabbits when healthy caecal microflora is not yet established and juvenile rabbits are susceptible to disease. Ingestion of maternal caecotrophs aids the population of the caecum with a healthy gut flora and early weaning and separation from the dam increase susceptibility to bacterial enteritis.
After weaning, ammonia levels in the caecum decrease as the diet changes. Caecal pH becomes more acidic as volatile fatty acid concentrations increase. The proportions of individual volatile fatty acids alter with the change of diet from milk to solids. Propionate, valerate and branched chain fatty acids predominate until the rabbit starts to eat solid food. The microbial flora changes in association with alterations in volatile acid production (Padilha et al., 1995). Pathogenic strains of E. coli, Clostridium spp., coccidiosis or rotaviruses are likely to be present in the environment of newly weaned rabbits. Several animals sharing a small space increase faecal contamination and the risk of cross-infection. After weaning, rabbits are often stressed by change of housing and diet, transport and mixing with different individuals. Intercurrent disease such as pasteurellosis can be present. Minimizing stress and a diet containing sufficient indigestible fibre is especially important at this age to prevent enteric disease.
In the adult pet rabbit, infectious causes of enteritis are rare in comparison with the young commercial rabbit. Instead, it is far more common to see diet-related problems. Dietary changes can result in the production of soft caecotrophs that are left uneaten. Instead, the caecotrophs are deposited in the bedding or are found stuck to the fur under the tail. The smelly faecal mass is often mistaken for ‘diarrhoea’ by the owner, although it is not caused by infection (see Section 8.6).
8.2.4 Effect of digestive disease on water and electrolyte exchange
The effects of gastrointestinal disease on the water, electrolyte and acid–base balance of rabbits are complex. Disturbances in water, electrolyte and acid–base balance have a rapid and profound effect on health. Water and electrolytes are continually exchanged along the digestive tract and, in rabbits, any condition that affects this cycle of secretion and absorption also affects fluid and electrolyte balance (see Figure 1.4). Saliva is constantly produced by rabbits and, during the hard faeces phase, water is secreted into the stomach and proximal colon. It is then reabsorbed from the caecum and distal colon. Dehydration develops rapidly during intestinal disease, despite no obvious fluid loss in vomit or diarrhoea. Conditions that cause intestinal obstruction result in the accumulation of large amounts of fluid proximal to the site of obstruction. Conversely, gastrointestinal hypomotility reduces the secretion of water into the stomach and results in impaction of the contents. Dehydration is associated with gastrointestinal hypomotility, presumably due to decreased absorption of water from the stomach, caecum and distal colon.
Mucus is rich in potassium (Riley and Cornelius, 1989) and a feature of enteropathy in rabbits is the production of large amounts of mucus. Diarrhoea can result in hypokalaemia (Licois et al., 1978).
The absorption and secretion of electrolytes along the digestive tract is also affected by changes in acid–base status. In a study by Charney et al. (1983), alkalosis in rabbits decreased the absorption of water, sodium and chloride, whereas acidosis had the opposite effect and reduced bicarbonate secretion. Anorexia in rabbits can quickly lead to metabolic acidosis (see Section 8.3.2) and the limited ability of the rabbit kidney to correct acid–base disorders makes the species vulnerable to the effects of acidosis or alkalosis (see Section 1.13.12). Changes in acid–base status can affect the contractility of the proximal colon (Lofqvist and Nilsson, 1981), which, in turn, will affect the secretion of water and its absorption from the caecum.
Therefore, effective fluid therapy is a vital part of the treatment of many gastrointestinal diseases in rabbits (see Section 3.11). Decisions on what fluid to administer should be based on electrolyte status determined by blood sampling. Intravenous or intraosseous fluid therapy is necessary for most cases. Although subcutaneous fluids can be used, they are not suitable for ill, dehydrated or hypotensive patients as absorption of fluids from under the skin is poor when peripheral tissue perfusion is reduced by shock or hypovolaemia.
8.3 Gastrointestinal hypomotility
8.3.1 Gastrointestinal hypomotility and formation of trichobezoars (hairballs)
Optimum gastrointestinal motility is important for digestion of food, absorption of water and electrolytes and maintenance of a healthy gut flora. Many factors influence gastrointestinal motility in rabbits (see Box 8.1). Reduced gastrointestinal motility leads to impacted food in the stomach or caecum, impaired glucose absorption and a reduction in the supply of nutrients and fluids to the caecal microflora.
For many years, the presence of impacted hair and food material in the stomach (‘trichobezoars’ or ‘hairballs’) was believed to be the cause of disease in rabbits. It was thought that the trichobezoar caused a pyloric obstruction. Anorexia, weight loss, reduced faecal output, depression and death due to starvation were attributed to the presence of a trichobezoar. Many theories were put forward about the cause of trichobezoar formation. Handbooks and leaflets on rabbit care suggest that regular grooming is required to prevent excessive amounts of hair being swallowed and becoming impacted in the stomach. Some breeders still recommend one day a week without food for rabbits in order to ‘clear the system’ of ingested hair. Boredom, magnesium or copper deficiency, inadequate protein, individual caging and the presence of air filtration barriers have all been put forward as potential causes of trichobezoar formation (Ojerio and Ladiges, 1981). The rabbit’s inability to vomit has also been cited as a contributory factor (Gillett et al., 1983). Treatment was usually unsuccessful. The administration of liquid paraffin to lubricate the gastric contents and that of pineapple juice to dissolve the hair enzymatically with bromelain were suggested as therapies. Surgical removal of the trichobezoar was a last resort and carried a poor prognosis. The association between trichobezoars and fatty liver was noted by many authors (Gillett et al., 1983; Ojerio and Ladiges, 1981). An association with pregnancy toxaemia was made by Patton et al. (1983). It is only in recent years that trichobezoars have been recognized as the result of anorexia rather than the cause. On post-mortem examination, the rabbit’s stomach is never found to be empty (Okerman, 1988) and the presence of a small amount of fibrous food is normal. The presence of hair entangled in the food is also a normal finding because rabbits are continually grooming and ingesting large amounts of hair.
In 1984, Leary et al. attempted to induce the clinical syndrome associated with the presence of trichobezoars by the orogastric infusion of latex to reproduce a gastric foreign body. Monthly radiographs were taken and the rabbits monitored closely for food intake and faecal output for 24 weeks after infusion. Gastrotomies were then performed to remove the foreign material and the rabbits monitored closely for a further 4 weeks prior to euthanasia and post-mortem examination. The presence of a latex bezoar did not have any adverse effect on appetite and weight gain of any of the 12 rabbits that were infused. In the same study, the stomach contents of 208 clinically healthy commercial rabbits were examined after slaughter and well-defined trichobezoars were found in 23% of them. This study cast doubt on the concept that trichobezoars cause anorexia. In 1986, Fekete and Bokori found elevated cortisol levels in rabbits with trichobezoars, although they concluded that the elevation was associated with the stress of having a trichobezoar rather than the trichobezoar being the result of stress. In 1987, Buckwell described the successful medical treatment of ‘gut stasis’ in rabbits exhibiting anorexia, reduced water intake, depression, weight loss and absence of faecal pellets. He described the presence of a palpable impacted mass in the region of the stomach. Treatment consisted of the administration of a motility stimulant, corticosteroid, oral fluid and the provision of hay. Since that time, trichobezoars have increasingly become recognized as the result, rather than the cause, of reduced gastrointestinal motility and are secondary to many other conditions.
Pain, stress and fright can all reduce gastrointestinal motility and lead to the accumulation of hair in the stomach and the formation of trichobezoars. In one study by Jackson (1991), intestinal stasis occurred more frequently in a group of laboratory rabbits that were restrained without the use of a towel. Once towel wrapping was introduced for the restraint of all rabbits, the incidence of the trichobezoars fell dramatically and the author concluded that stress played a significant role in the cause of the disease. Stimulation of the sympathetic nervous system causes adrenal hormones to be released into the circulation. One of the effects of adrenaline and noradrenaline is the inhibition of gastrointestinal motility. If gastrointestinal motility is reduced, water secretion into the stomach is also reduced and hair and ingesta accumulate and become impacted. The rabbit appears to be particularly susceptible to the effects of catecholamines on gut motility. In healthy rabbits with an uninterrupted daily routine, the passage of soft and hard faeces follows a circadian rhythm, with an average day-to-day variation of approximately 30 minutes. Stress or even alterations in routine can have a significant effect on the caecotrophic rhythm. Simply switching a light on during the normal period of darkness caused one rabbit to stop producing faeces for 10 days in a study of caecotrophic rhythm by Jilge (1980). Thunderstorms, bonfire night, predator attacks, pain and surgery can all slow gut motility and, if left untreated, result in impacted stomach contents and trichobezoar formation.
Gut motility is affected by the indigestible fibre component of the diet. The provision of a high fibre diet has long been recognized as a preventative measure for the formation of hairballs (Sandford, 1996). Rabbits fed on a low fibre diet are at greater risk of developing gastric stasis and trichobezoar formation. Rabbits with slow gut motility crave fibre and will often eat hay or grass in preference to other foods. The provision of palatable indigestible fibre for rabbits that are at risk of gut stasis, e.g. postoperatively, is important. Fresh grass is the most acceptable form of fibre for rabbits, although good-quality hay is acceptable.
Slow gut motility not only results in the development of trichobezoars. Gas accumulates in the stagnant stomach and caecum. Visceral distension causes pain that stimulates catecholamine release and exacerbates inhibition of gut motility. Gastric ulceration can occur. Alterations in the secretion and absorption of water and electrolytes cause dehydration and electrolyte imbalances. Reduced food intake leads to an energy deficit that stimulates mobilization of free fatty acids from adipose tissue and fatty infiltration of the liver. Ketoacidosis and hepatic lipidosis are the result. Liver failure from hepatic lipidosis is the usual end-point of untreated gastrointestinal stasis.
Reduced food intake and hypomotility of the proximal colon also reduce the amount of ingesta available as substrate for caecal microflora. Alterations in the caecal fermentation patterns can lead to changes in caecal pH and volatile fatty acid production. The balance of caecal microflora changes with the possibility of the proliferation of pathogenic bacteria such as Clostridium spp.
8.3.2 Anorexia and development of hepatic lipidosis
Anorexia, whatever the cause, can trigger a chain of events that can result in death of a rabbit from hepatic lipidosis and liver failure. Rabbits are obligate herbivores and their carbohydrate metabolism differs from carnivorous species such as the dog or cat. The endocrine control of the storage and mobilization of food are not as important in herbivorous species as in carnivores that eat periodically and need to regulate a fluctuating supply of nutrients from the digestive tract. Herbivorous animals withstand the absence of insulin far more readily than carnivorous ones (Bentley, 1998).
In rabbits, glucose and lactates are produced in the caecotrophs during the period of fermentation in the stomach and are absorbed during digestion of caecotrophs in the small intestine. Amylase is synthesized by the caecal flora and is present in caecotrophs to act on the carbohydrates that are present. Volatile fatty acids are a major energy source and represent about 40% of the maintenance energy requirement of rabbits (Marty and Vernay, 1984). They are absorbed from the digestive tract during digestion of caecotrophs and from the caecum where volatile fatty acids are produced by bacterial fermentation. Concentrations of volatile fatty acids in arterial blood are kept constant by the liver, despite fluctuations in absorption from the digestive tract. Volatile fatty acid absorption from the gut varies with the hard or soft phase of faecal excretion (Vernay, 1987) and is affected by gut motility. Lipids are absorbed from the diet or are derived from endogenous synthesis of free fatty acids in the liver (Madry et al., 1976).
During periods of anorexia, glucose absorption from the gut falls and there is a reduction in volatile fatty acid production by the caecal microflora. The resultant drop in glucose and glucogenic volatile fatty acid absorption results in hypoglycaemia, which stimulates lipolysis and the mobilization of free fatty acids from adipose tissue. The free fatty acids are transported to the liver to be metabolized as an energy source. The major pathway for their degradation is that of β-oxidation and ketone body production. Ketoacidosis occurs when ketone body production exceeds tissue metabolism. Rabbits do not have effective metabolic pathways for correcting acidosis (see Section 1.3.12) and are particularly susceptible to the effects of ketoacidosis. Also, during periods of increased mobilization of free fatty acids, a ‘bottleneck’ develops in the liver, which impairs the metabolic pathways that result in lipid transport to other tissues. Fat accumulates in the hepatocytes, causing cholestasis and eventual liver failure and death. Hepatic lipidosis occurs most readily in obese rabbits because they have already accumulated triglycerides in the hepatocytes.
8.3.3 Obesity, pregnancy toxaemia and hepatic lipidosis
Any condition that causes prolonged anorexia in rabbits can result in fatty infiltration of the liver. Stress alone alters the fat metabolism of rabbits, especially in those that are already overweight. In a study by Lafontan and Agid (1979), minor stressful stimuli such as saline injections or venepuncture induced a prompt increase in plasma free fatty acid and glycerol concentrations in naturally obese rabbits. This increase did not occur in younger, lighter rabbits. α-Adrenergic responsiveness is increased in the large fat cells of obese rabbits in comparison with the small fat cells of underfed rabbits (Lafontan, 1981). High fat diets greatly increase the risk of hepatic lipidosis. In a study by Jean-Blain and Durix (1985), rabbits fed on a high fat diet showed a twofold increase in ketonaemia during a period of fasting and were more hypoglycaemic than rabbits fed on a low fat diet. Obesity is a major problem in pet rabbits and animals that already have fatty livers can rapidly develop hepatic lipidosis if they become anorexic. Any surgical procedure in an obese rabbit carries a risk of hepatic lipidosis associated with pain, stress and withholding food.
It is well known among rabbit breeders that female rabbits should not be too fat if they are to breed successfully. Rabbits with fatty livers readily develop ketosis (pregnancy toxaemia) in late pregnancy or during early lactation when glucose requirements are high. Healthy pregnant rabbits show a period of insulin resistance between days 24 and 30 of gestation, during which high levels of insulin do not stimulate muscle glucose uptake (McLaughlin and Fish, 1994). Hepatic uptake of glucose and free fatty acids is also reduced in pregnant rabbits and brings about an arterial hyperglycaemia so more glucose is available to the uterus (Pere et al., 1992). Insulin resistance results in increased hormone sensitive lipase (HSL) activity, with increased amounts of triglyceride being hydrolysed from adipose tissue and transported to the liver.
Pregnant does are susceptible to the effects of anorexia and a drop in blood glucose rapidly stimulates fatty acid mobilization from adipose tissue to a liver already being compromised by fatty infiltration. An association between hairballs and pregnancy toxaemia was made by Patton et al. (1983), illustrating the complex inter-relationship between anorexia, fibre, gastrointestinal motility, energy demand and fatty infiltration of the liver. Treatment of pregnancy toxaemia is unlikely to be successful, but follows the same principles as the treatment of hepatic lipidosis, apart from the complication of the fetuses. Pregnancy toxaemia can be prevented by keeping breeding does slim and feeding them a high fibre diet.
8.3.4 Diagnosis and treatment of gastrointestinal hypomotility and prevention of hepatic lipidosis
Hepatic lipidosis can be prevented in anorexic rabbits by maintaining a positive energy balance with nutritional support and prompt treatment. It is important that rabbit owners are made aware that anorexia, in conjunction with lack of faecal output, is a potentially fatal condition and that rabbits that are not eating must be treated promptly. Hospitalization and intensive nursing are often needed.
There are many differential diagnoses for the underlying causes of anorexia and gastrointestinal hypomotility, including dental disease and recent surgery (see Table 1.7). Recognition and treatment of the underlying cause is an essential part of treatment. The onset of gastrointestinal hypomotility can be insidious and anorexic rabbits are often reasonably alert in the early stages. A reduced appetite and a reduction in faecal output are the early warning signs (Table 1.6). As the disease progresses, the rabbit becomes totally inappetent and depressed. It adopts a hunched appearance and may sit for hours, immobile in the corner of the cage or hutch. Affected rabbits do not groom and appear to be oblivious to their surroundings. They are no longer inquisitive and do not respond to being spoken to or the offer of an interesting titbit. The rabbit becomes clinically dehydrated. There are no specific clinical signs associated with the development of hepatic lipidosis but affected animals are depressed and unresponsive. In the terminal stages, they become totally inappetent and are often disorientated and ataxic. Hyperglycaemia occurs. Death is due to liver and kidney failure.
Diagnosis of gastrointestinal hypomotility can be made on clinical history and examination. It can be confirmed by radiography (see Box 8.2 and Figures 8.3, 8.4 and 8.5). Faecal output ceases completely and the impacted stomach can often be palpated as a hard mass behind the ribs, especially in the later stages of the disease. A blood sample can aid differential diagnosis, assist with choice of fluid therapy and offer prognostic indicators. A lipaemic sample or the presence of hyperglycaemia in conjunction with ataxia is a poor prognostic sign. In the early stages, hypoglycaemia may be found. This is treated by oral, subcutaneous or intravenous glucose therapy. In contrast, some rabbits show hyperglycaemia in the early stages of the disease associated with stress or pain. A blood glucose value within the normal reference range is reassuring. A PCV in excess of 40–45% indicates dehydration. Prerenal azotaemia is common in rabbits with gastrointestinal stasis. It is found in conjunction with dehydration. Blood urea and creatinine levels can be markedly elevated. If the analytical equipment is available, electrolyte status is invaluable. Once hepatic lipidosis is established, fatty infiltration of the kidneys occurs and the rabbit goes into liver and kidney failure. There can be a range of bizarre biochemistry results at this stage.
Table 8.3
Properties and dosages of therapeutic agents used in the treatment of enteric disorders of rabbits
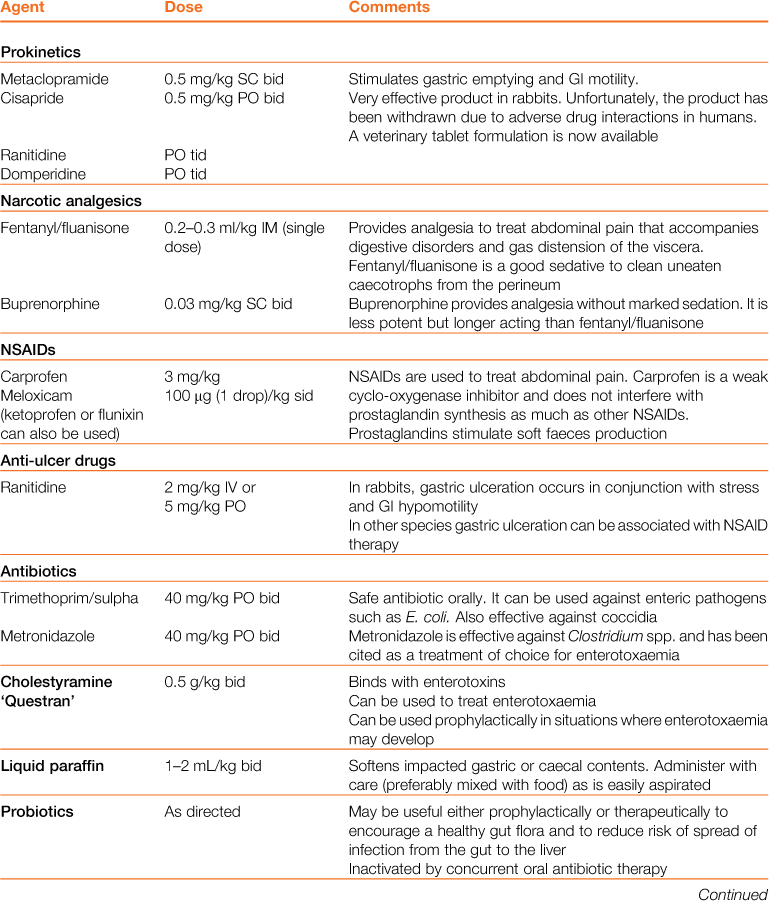
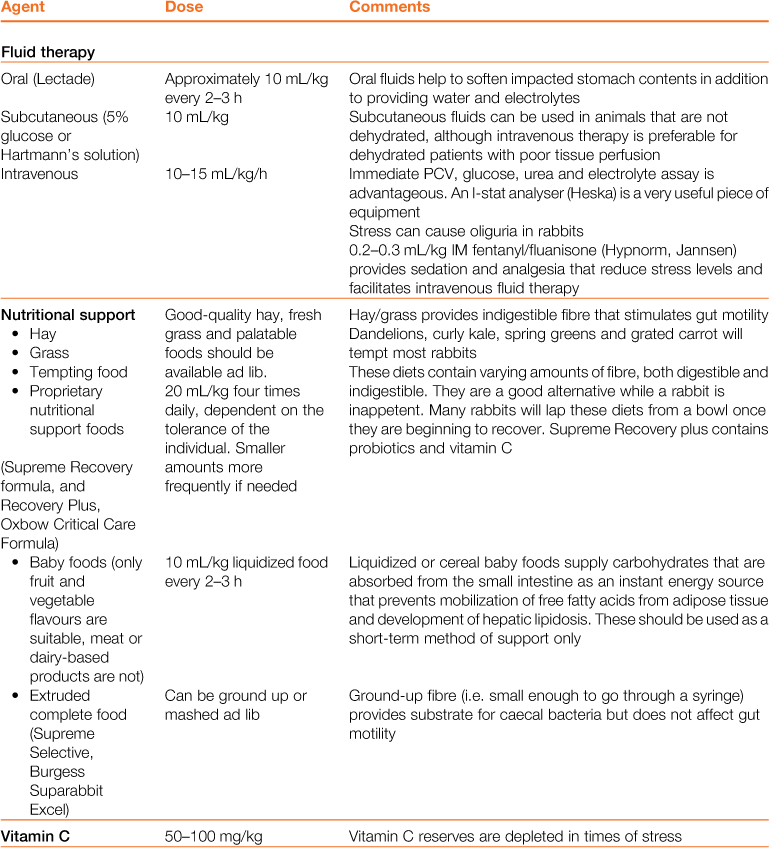
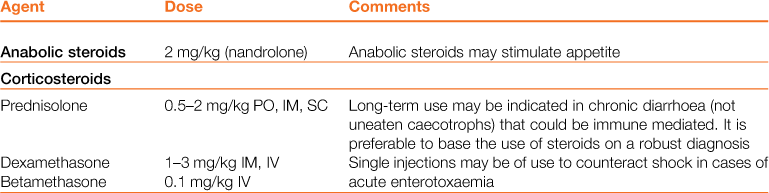
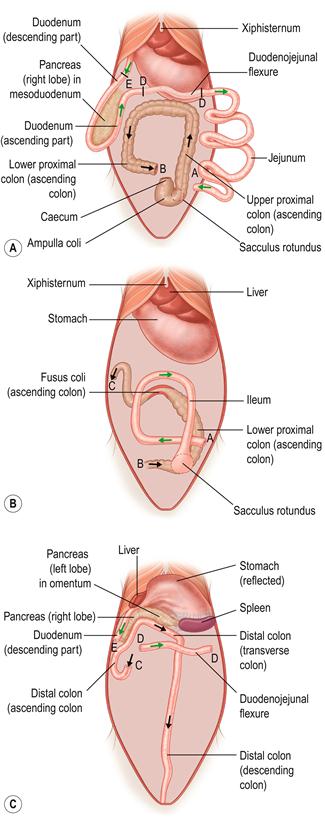
Figure 8.2 Three-dimensional topographical anatomy of the abdominal contents of the rabbit with the caecum removed.
The topographical relationship of the liver, spleen, pancreas, small intestine and colon at three levels from superficial (ventral, A) to deep (dorsal, C. The mid-abdomen is illustrated in B) is illustrated. The diagrams were drawn from fresh dissections after removal of the caecum. Each of the three drawings shows the small intestine in green and the large intestine in black. Dotted lines show structures that are deeper (more dorsal) than the illustrated layer. The progression through the bowel from stomach to anus is shown by arrows. The intestines are held firmly in place by their mesenteric attachments.
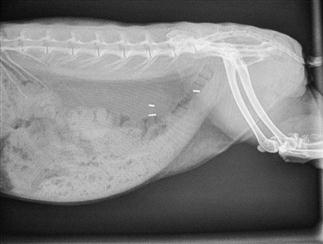
Figure 8.3 Radiographic anatomy of lateral view of normal abdomen.
Interpretation of abdominal radiographs is summarized in Box 8.2. A lateral radiograph of a healthy 2-year-old neutered female rabbit is shown. Three surgical clips are visible in the caudal abdomen, used during ovariohysterectomy. The radiograph was taken during the hard faeces phase, and hard pellets are visible in the rectum. The ileocaecocolic complex is visible in the caudoventral abdomen; it is moderate in size and its contents are amorphous. Both kidneys, the stomach, liver and bladder can be visualized.
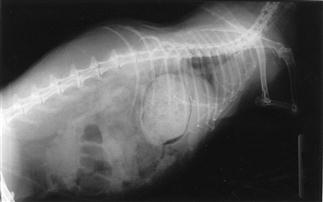
Figure 8.4 Oblique view of a rabbit with gastrointestinal hypomotility showing presence of a trichobezoar (hairball).
An oblique view of a 3-year-old, obese female rabbit with gastrointestinal hypomotility is shown. Gas shadows are evident in the stagnant caecum and stomach. The stomach contains a mass of impacted food and hair that has resulted from a decrease in gastrointestinal motility (see Section 8.3.1). The initiating cause was unknown; the rabbit was presented in a moribund state and subsequently died. Post-mortem examination confirmed the presence of hepatic lipidosis. There was also fatty degeneration of the kidneys.
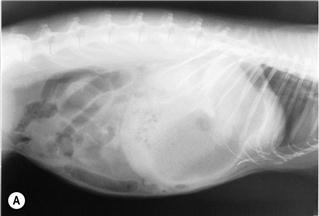
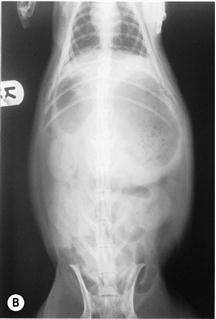
Figure 8.5 (A) Lateral view of a rabbit with an intestinal obstruction.
A lateral view of a 2-year-old dwarf lop male rabbit with an acute intestinal obstruction caused by a felt of ingested hair is shown. The radiograph shows a grossly distended stomach containing fluid and gas. There is gas distension of the small intestine proximal to the site of obstruction that was in the ileum. This is a characteristic radiograph. The foreign body was surgically removed promptly (see Section 8.5.1) and the rabbit made an uneventful recovery. A differential diagnosis of intestinal obstruction is mucoid enteropathy, in which gastric dilatation may be seen in the terminal stages. An impacted caecum that can be palpated or seen radiographically is generally associated with mucoid enteropathy (see Figure 8.9).
(B) Dorsoventral view of a rabbit with an intestinal obstruction.
Figure 8.4 shows the same rabbit as Figure 8.5. Knowledge of the topographical anatomy of the intestines aids diagnosis and location of a foreign body (see Figure 8.2). The radiographs here in (A) and (B) were taken after sedating the rabbit with 0.2 mL/kg fentanyl/fluanisone (see Box 4.6). It is possible to obtain a diagnostic radiograph by placing a conscious rabbit in ventral recumbency on an X-ray plate, although positioning will be poor because the hind legs cannot be extended. Hypnosis and non-manual restraint with sandbags can be used to obtain a lateral view. A quiet room, gentle handling and patience are needed. The rabbit can be kept calm by covering its head with a towel.
Treatment of gastrointestinal hypomotility is aimed at restoring appetite, correcting electrolyte imbalances, correcting dehydration, stimulating gastric emptying, promoting normal gastrointestinal motility and softening and lubricating impacted food and hair. The medical treatment of gastrointestinal hypomotility and the properties of therapeutic agents are summarized in Tables 8.2 and 8.3. The general treatment of digestive disorders is given in Box 8.3. Nutritional support is important to prevent the development of hepatic lipidosis. Analgesics are always indicated, as gas accumulates in stagnant sections of the gastrointestinal tract, causing distension and pain, which compound the situation further.
Diet is a key part of the treatment of gastrointestinal hypomotility, and nutritional support will prevent the development of hepatic lipidosis. All anorexic rabbits must be encouraged to eat, and assist fed if they will not or are unable. Hepatic lipidosis can develop in any rabbit that becomes anorexic, although the risk is greater in obese, pregnant or lactating animals. Tempting foods such as fresh grass, dandelions and appetizing vegetables such as curly kale, spring greens, carrots and apples should be offered. Good-quality hay is important, both to stimulate appetite and to provide a sense of security to reduce stress levels. A bed of hay smells familiar. Grass and hay provide long particles of indigestible fibre that are important to stimulate gut motility. A quiet environment away from predators and barking dogs is important. In the initial stages (less than 24 h), analgesia and the provision of palatable fibre can be sufficient to stimulate gut motility and prevent progression of the disease. In the later stages (more than 24 h without food), syringe feeding is required to provide calories and fluid to soften and lubricate impacted stomach contents and provide water and electrolytes. Several proprietary brands of support feed are available for herbivores, and these should be fed as per the manufacturer’s instructions. Puréed vegetables or baby foods provide an easily assimilated digestible energy source that can be given through a syringe, although these are not sufficiently high in fibre. A source of fermentable fibre is important to provide nutrients for caecal bacteria. Indigestible fibre is difficult to administer through a syringe because the large particles clog the nozzle; however, purpose-made support foods do contain some. There is no point in attempting to grind indigestible fibre down for syringe feeding in an attempt to stimulate gut motility. Grinding fibre to a particle size where it no longer clogs a syringe means that the particles are small enough to be moved into the caecum instead of the colon and the stimulatory action on the gut is lost. Nasogastric tube feeding may be necessary as a last resort for intractable cases, but nasogastric tubes can be counterproductive. They clog up easily and an Elizabethan collar is required. Elizabethan collars have been proven to be stressful to rabbits (Knudtzon, 1988). Pharyngostomy tubes are somewhat more practical and are placed in much the same way as they are in cats. They have the advantage that being wider bore they are less likely to block; however, it is still difficult to get sufficiently fibrous food through. Pharyngostomy tubes also carry the risk of causing abscessation at the site of entry. The use of PEG tubes has been reported; however, this is not commonly done due to the risk of abscessation at the entrance site and the difficulty of inserting these in the rabbit. All three types of feeding tubes carry common disadvantages: they are unable to deliver food that will promote gut motility; they do not promote tooth attrition; and they have risks associated with their insertion. They are, however, a means to an end where otherwise a patient may die. Total parenteral nutrition has also been reported occasionally in rabbits; however, this is unlikely to be available outside of large referral institutions and is associated with the risks of sepsis, anaphylaxis, cholestasis and inflammation of the gut lining.
Pineapple juice or proteolytic enzymes have been recommended as remedies for hairballs because they are reputed to dissolve hair. Miller (1983) conducted an experiment in which they incubated rabbit hair for up to 3 days in papaya, proteolytic enzymes or pineapple juice. The pH of the solution was adjusted to 2 with hydrochloric acid to mimic conditions in the rabbit stomach. They found no difference between the treated and untreated control samples and the authors concluded that none of the enzyme treatments exhibited any ability to dissolve hair. The success of pineapple juice as a remedy for gastric stasis might be due to the introduction of liquid into the stomach that softens the hairball and aids its passage out of the stomach. Liquid paraffin can be used to soften and lubricate impacted stomach contents.
Motility stimulants are effective in promoting gastrointestinal motility. Cisapride is a very effective remedy for gastrointestinal hypomotility (see Section 3.7.1); however, because of adverse drug interactions in humans, it was withdrawn from many countries in 2004 and has been used much less in the treatment of gut stasis in the past 10 years. Many clinicians have not felt that treatment was less successful without cisapride, and have not reverted to its use now that it can be legally obtained once more. Metoclopramide is an alternative therapy but appears to be less effective than cisapride. Atropine and opioid analgesics can antagonize the effects of metoclopramide. There is in vitro evidence that metoclopramide is only effective in adult rabbits; however, anecdotally this does not appear to be the case (see Section 3.7.2).
Fluid therapy is always indicated in rabbits in the later stages of gastrointestinal hypomotility. Oral or subcutaneous fluids might be sufficient if the rabbit is not clinically dehydrated but intravenous therapy or intraosseous fluid therapy is essential once dehydration becomes evident.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree