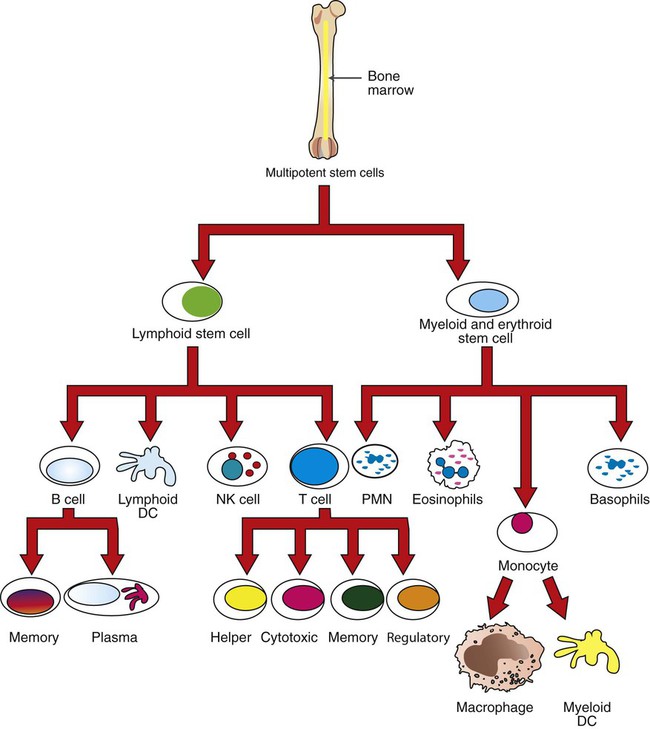
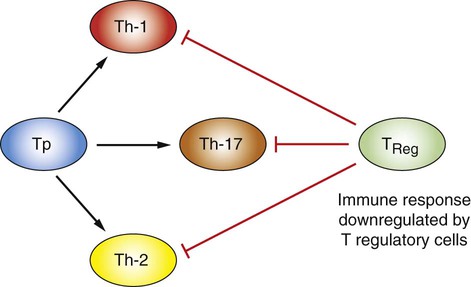
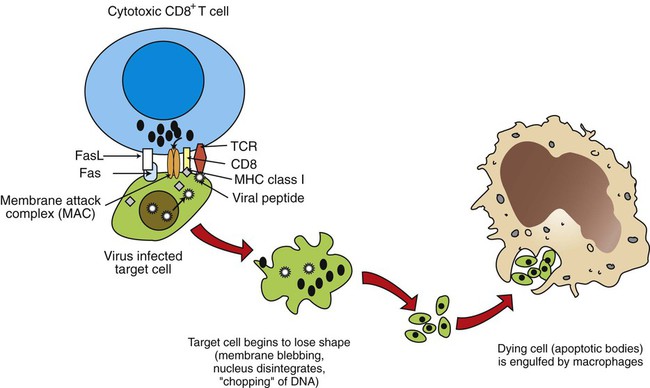
1. Mature T cells develop from lymphoid stem cells that have migrated to the thymus. 2. T cells are a heterogeneous population of cytotoxic T cells and T-helper cells. Interactions of Antigen-Presenting Cells and T Cells 1. Major histocompatibility complex proteins are considered the central regulators of the immune system. 2. MHC class I antigens of infected nucleated cells play a major role in activating cytotoxic T cells. 3. MHC class II antigens on antigen-presenting cells play a major role in selective activation of T-helper cells. 1. Initial exposure to foreign antigen induces slow onset of antibody appearance, whereas subsequent exposure induces faster, longer-lasting antibody appearance. 2. Antibodies, or immunoglobulins, are glycoprotein molecules that can be divided into five isotypes, or classes. 3. The B-cell population produces antibodies to millions of different antigens, yet the antibody-antigen interaction is specific. 4. Expansion of an antigen-specific B–memory cell population on initial antigen exposure results in a faster, more pervasive, secondary immune response. Regulation of Immune Responses 1. The actions, secretions, and surface molecule expression of immune cells play an important role in regulation of the body’s immune response. As discussed in Chapter 54, innate immunity offers effective defense against a wide range of pathogens. Key features of innate immunity include (1) rapid response against invading pathogens; (2) nonspecificity; and (3) physical, chemical, and cellular (phagocytic cells, NK cells) barriers. The response of the innate immune system, however, is not long-lasting and does not induce immunological memory (i.e., ability to recall previous exposure to antigens and respond to these effectively and specifically). For long-lasting immunity, another arm of the immune system must be activated. This is referred to as acquired immunity, which involves activation of T and B lymphocytes. Antigen-presenting cells (APCs), a part of the innate immune system, play a central role in activating lymphocytes. Activated T lymphocytes (T cells) secrete cytokines that are essential for defense against intracellular pathogens, activation of other cells, and coordination of immune responses. B lymphocytes (B cells) have two main functions: (1) secreting antibodies that bind specifically to the antigen that induced the antibody response and (2) acting as APCs. Before discussing how antigens are presented to specific lymphocytes, it is important to understand the different types of immune cells (Figure 55-1). All cells of the immune system are derived from multipotent stem cells that are located primarily in the marrow of long bones. These multipotent stem cells subsequently give rise to primordial stem cells, such as lymphoid stem cells or myeloid stem cells. Myeloid stem cells give rise to monocytes, which mature in tissues to become macrophages or dendritic cells. Lymphoid stem cells give rise to T, B, natural killer (NK), and lymphoid dendritic cells. Mature cells are found circulating throughout the body but concentrate in the peripheral lymphoid organs (e.g., lymph nodes, spleen) and gut-associated lymphoid tissues, where most of the complex interactions with antigens take place. T-helper cells, based on the predominant cytokines secreted, are further divided into three major types: Th-1, Th-2, and Th-17. Th-1 cells predominantly secrete interleukin-2 (IL-2), interferon-gamma (IFN-γ), and tumor necrosis factor beta (TNF-β). Th-1 immunity is critical for defense against intracellular pathogens (viral, bacterial, or protozoal) and certain types of tumors. Th-1 cells are preferentially generated when naïve CD4+ cells are exposed to IL-12, a cytokine from antigen-presenting cells (Figure 55-2). Failure to generate Th-1 cells creates susceptibility to these infections. Abnormal activation of Th-1 cells can result in a wide variety of inflammatory conditions, including autoimmune states. Activation of naïve CD4+ cells with interleukin-4 (IL-4) leads to differentiation into Th-2 cells (see Figure 55-2). Th-2 cells predominantly secrete IL-4, interleukin-6 (IL-6), interleukin-5 (IL-5), and interleukin-10 (IL-10). Generation of Th-2 cells is essential for defense against extracellular pathogens, neutralization of toxins and viruses in body fluids, and activation of other cells of the immune system. Abnormal regulation of Th-2 cells leads to allergies. Activation of CD4 T-helper cells with IL-6 and transforming growth factor beta (TGFβ) induces the differentiation of Th-17 cells, which secrete a powerful pro-inflammatory cytokine, interleukin-17 (IL-17; see Figure 55-2). This cytokine is now recognized as an important mediator of inflammatory and autoimmune diseases. IL-17 acts on target cells to activate key signaling molecules to promote inflammation through several mechanisms including: (1) recruiting inflammatory cells (e.g., neutrophils, monocytes, and macrophages) to the site of inflammation; (2) acting on target cells (e.g., fibroblasts, epithelial cells) to stimulate a broad range of strong pro-inflammatory molecules (e.g., IL-6, monocyte chemotactic protein 1, nitric oxide); and (3) synergizing with TLR ligands. Although IL-17 is protective in infection, overproduction of IL-17 is known to aggravate certain disease conditions (e.g., autoimmune diseases such as systemic lupus erythematosus and multiple sclerosis). Exposure of CD4 naïve cells to TGFβ alone (e.g., in the absence of IL-6) will drive the differentiation to T regulatory cells (Treg cells; see Figure 55-2). Treg cells are a population of T cells which act as powerful suppressors of the T cell-mediated immune response and of self-reactive T cells in autoimmune diseases. Tregs utlilize a broad range of suppressive mechanisms which include release of immunosuppressive cytokines TGFβ and IL-10, and cell-cell contact. IL-10 and TGFβ secreted by Tregs are critical for dampening immune responses in allergy, burns, pregnancy, cancer, viral diseases, and autoimmune diseases. Treg cells can inhibit and downregulate all three (Th-1, Th-2, and Th-17) subsets of CD4 cells (see Figure 55-2). Dysregulation of Treg cells can lead to massive inflammatory diseases, while excessive numbers or functions of Tregs can lead to dampening of immune responses that lead to severe infections. Therefore, physiologically, Treg cells must be finely balanced to maintain immune health status. Cytotoxic killing of intracellularly infected, cancer, or autoreactive cells is an essential step in survival by containing infected cells or the spread of deleterious cells. For example, a viral infection of any cell in the body yields to viral replication within the cell, and some of these viral peptides will physically bind to intracellular MHC class I antigens (Figure 55-3). This viral peptide–MHC class I complex is carried to the surface and displayed as an altered MHC class I molecule. TCR molecules of effector CD8+ cytotoxic T cells will recognize the class I molecule–peptide complex to initiate cytotoxicity by at least four different, but complementary, mechanisms. First, contact of a cytotoxic CD8+ cell with an infected cell that is displaying a MHC class I–peptide complex will immediately result in cytoplasmic reorganization within the CD8+ cell. This includes the alignment of granules and Golgi apparatus at the site of contact. Perforins in the cytotoxic cells polymerize to form tiny injectable tubes referred to as membrane attack complexes (MACs) that “drill” holes into the target cells. Granzymes are passed from the cytotoxic cells into the target cells through these perforin tubes to initiate apoptosis.
The Specific Immune Response
Acquired Immunity
T Cells (T Lymphocytes)
Mature T Cells Develop from Lymphoid Stem Cells That Have Migrated to the Thymus
T Cells Are A Heterogeneous Population of Cytotoxic T Cells and T-Helper Cells
Interactions of Antigen-Presenting Cells and T Cells
Major Histocompatibility Complex Proteins Are Considered the Central Regulators of the Immune System
MHC Class I Antigens of Infected Nucleated Cells Play A Major Role in Activating Cytotoxic T Cells
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Specific Immune Response: Acquired Immunity
Only gold members can continue reading. Log In or Register to continue