CHAPTER 19 The Pancreas
Currently, no single test provides conclusive discrimination between inflammatory, cystic, neoplastic, and infectious diseases involving the pancreas. Patients with pancreatic disorders, with the exception of pancreatic insufficiency, often have similar histories and clinical signs. Clinicopathologic testing can frequently identify the presence of pancreatic disease in the dog; however, in the cat, serum chemistry tests are often less useful.1–3 In both dogs and cats, abdominal ultrasound is a useful diagnostic tool to visualize and assess an abnormal pancreas. As with biochemical tests, however, the use of abdominal ultrasound to definitively distinguish between pancreatic diseases has variable sensitivity and specificity.2,4 Once visualized, ultrasound-guided fine-needle biopsy (FNB) of the pancreas is a safe and effective adjunct to imaging in the diagnosis of pancreatic disorders. The pancreas exfoliates well, and cytologic examination of the pancreas in small animals has proved useful in the diagnosis of both neoplastic and nonneoplastic lesions, including abscesses, cysts, and pancreatitis.
NORMAL PANCREAS STRUCTURE
Anatomy and Histology
The pancreas consists of a right (duodenal) and left (transverse or splenic) limb joined at the head. The number and position of the pancreatic duct(s) opening into the duodenum and the location of the duct(s) to the common bile duct varies among species and among individuals in each species.5 The pancreas consists of endocrine and exocrine components. Numerous tubuloacinar secretory units form the exocrine component of the organ. These secretory units drain into long, narrow intercalated ducts lined by elongated, cuboidal cells. Intercalated ducts communicate directly with interlobular ducts.6 Functionally, the tubuloacinar secretory units (exocrine pancreas) secrete digestive enzymes in an inactive proenzyme form. Pancreatic enzymes are activated by trypsin secreted by the duodenum.
The endocrine islets of Langerhans are clusters of epithelial cells scattered among the secretory units. Normal pancreatic islets contain four cell types, each secreting different pancreatic polypeptides: Alpha cells secrete glucagon, beta cells secrete insulin, D cells secrete somatostatin, and F cells secrete pancreatic polypeptide. Beta cells are the most numerous islet cells (composing 60% to 70% of the islet cells) and they are generally concentrated in the central part of the islet. The alpha cells compose about 20% of the islet cells and are generally located peripherally.5
SAMPLING TECHNIQUE
Methods
In veterinary medicine, percutaneous, ultrasound-guided fine-needle aspiration (FNA) of the pancreas is the most common method of tissue sampling although intraoperative sampling can also be performed.7 FNB of the pancreas permits extensive sampling and, in people, is associated with a low risk of morbidity and mortality. It is especially useful in discriminating between pancreatic neoplasia and inflammation, with minimal complications. In human medicine, FNA is the diagnostic method of choice for patients with a pancreatic mass. The most common methods of sample procurement are computed tomographically guided or endoscopic ultrasound-guided aspiration. The vast majority of human pancreatic masses are neoplastic; as such, FNB is used to establish a rapid tissue diagnosis before chemotherapy and/or surgery.
Methods to obtain a FNB of the pancreas are outlined in Box 19-1. The described methods for sample procurement are closely based on methods originally published by Bjorneby and Kari.7 In brief, the pancreas and surrounding abdominal structures should be thoroughly evaluated with ultrasound to visualize the area to be aspirated. If a mass is present, multiple areas within the mass and surrounding tissue should be aspirated. In dogs, inflammation (purulent or lymphocytic) has been shown to occur in discrete areas throughout the pancreas (right and left limb).7 Therefore, there is no preferential site to sample the pancreas (and confirm pancreatitis) in the absence of a visible lesion. Label clean glass slides, preferably with frosted edges, with patient identification and site of aspiration. Draw 1 ml of air into a 3-ml syringe. Attach a 1½- to 3-inch 22-gauge needle to the syringe. This needle/syringe combination may permit more accurate needle placement and angle control.7 Using a guide or freehand, move the needle back and forth within the pancreas. Be careful to maintain the needle in the same tract. For sample procurement, no additional negative pressure is required. Do not attempt to redirect the needle because the tip of the needle may lacerate the tissue and cause excessive hemorrhage and leakage of pancreatic enzymes.7 To minimize cell disruption, sample expulsion and smear preparation should be as gentle as possible. To ensure full evaluation of the cells present, expel the sample onto the middle of the slide where cells are most readily stained and visualized. Sample three to four different sites within the lesion if possible. To ensure the best quality sample (and thus the likelihood of a cytologic diagnosis), make multiple smears using a variety of smear techniques that result in both thin and thick preparations. Slide preparation techniques include the squash smear (slide-over-slide) or blood smear technique. The smears should be air-dried and submitted to a veterinary clinical pathologist.7
Troubleshooting
In general, pancreatic tissue exfoliates well for FNB. If you have any concerns regarding sample quality, talk with your cytopathologist regarding sample attainment and preparation. Ruptured cells can be the result of negative pressure in the syringe while aspirating or too much pressure on slides while making preparations.7 Rapid drying of slides reduces refractile artifact on the slides. Hemodilution is common and generally will not confound diagnosis. However, if hemodilution is obscuring the diagnosis (especially distinguishing between blood contamination and inflammation), decrease the number of times the needle is moved within the pancreas. However, the trade off may be poor cytologic yield, which can occur if the needle biopsy technique is not aggressive enough. If clots tend to form, the needle and syringe can be flushed with an anticoagulant (i.e., ethylene-tetra-acetic acid [EDTA]) prior to organ aspiration. Nondiagnostic samples due to poor cellularity can occur when lesions are fibrous or if the lesion was missed during aspiration. Nondiagnostic samples should be interpreted in light of imaging findings. Reaspiration can always be attempted if the pancreas appears active and enlarged. However, if fibrosis appears possible, intraoperative biopsy will likely be superior for obtaining a diagnosis. Always interpret cytologic findings in light of imaging, physical examination, and biochemical findings. For example, if poorly cellular, proteinaceous fluid is obtained and the lesion on imaging is compatible with a cyst, then further diagnostics may not be warranted (Figure 19-1). However, if poorly cellular, proteinaceous fluid is obtained and the lesion is primarily solid or infiltrative with cystic or necrotic areas, then reaspiration may be indicated because the primary lesion may not be represented.
Complications
Significant adverse effects secondary to percutaneous FNB of the pancreas in dogs or cats have not been reported. Rarely, FNB of the pancreas, in human beings, has been reported to cause complications, such as needle tract seeding of tumors, fistula formation, and ascites.7 In one human study, complications arising from FNB of both solid and cystic lesions of the pancreas were noted in only 4 of 248 (1.2%) patients. These complications included acute pancreatitis and aspiration pneumonia and were noted only after aspiration of cystic lesions.8 Recommendations from this study included avoiding needle passage through the main pancreatic duct or branch ducts dilated proximal to an obstruction. In addition, aspiration was terminated if blood became visible in the syringe or if there was obvious hemorrhage within the target lesion.8
CYTOLOGIC EVALUATION
Normal
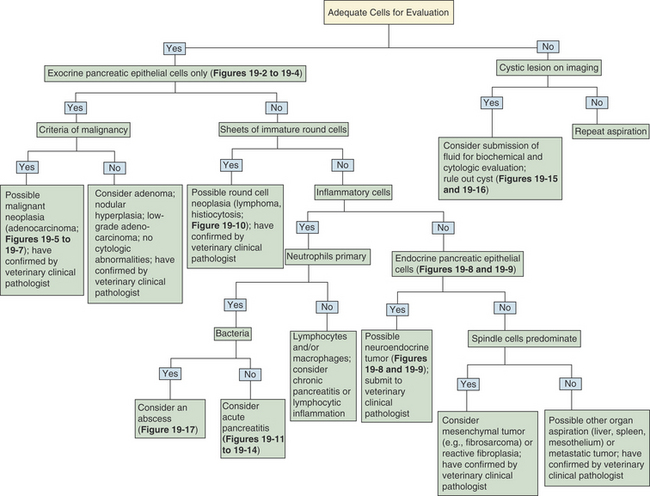
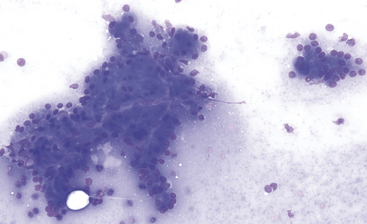
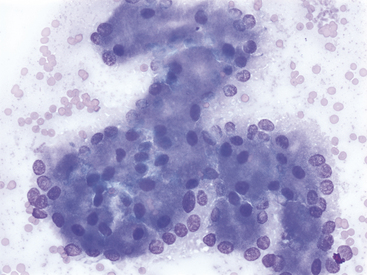
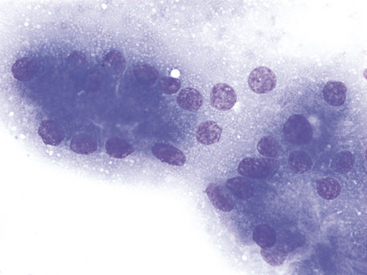
A decision tree to help guide initial cytologic evaluation of the pancreas is depicted in Figure 19-1. Exocrine epithelial cells are the most common cell type found on cytologic specimens from the pancreas. The background of the slidemay contain blood from iatrogenic contamination, or it may be light pink indicating a small amount of protein. Normal exocrine epithelial cells are found in small clusters to large sheets that may form tubular and acinar structures(Figure 19-2). On low magnification, the cytoplasm appears grainy with a pink hue due to the presence of small, pink granules. Unlike intestinal epithelial cells, cell-to-cell junctions are not prominent, giving cells a more indistinct, fluffy appearance (Figure 19-3). The cells are polyhedral with abundant cytoplasm and a low nuclear-to-cytoplasmic ratio (Figures 19-2 to 19-4). Nuclei are basilar in location, uniform, and round to oval. Chromatin is stippled to reticulate and a single, small, occasionally prominent nu-cleolus can be noted (see Figures 19-3 and 19-4). On high magnification, abundant pink, cytoplasmic granules, most consistent with membrane-bound zymogen granules, are noted. In preparations with abundant cell rupture, these granules can fill the background of the slide, giving a mottled blue and pink appearance (see Figure 19-4). In a FNB of normal pancreas, no other cell populations will be present in high numbers. Occasionally, hematopoietic precursors, indicative of extramedullary hematopoiesis, small ductal cells, or uniform endocrine epithelial cells, will be seen. The number of leukocytes present in the aspirate should be interpreted in light of peripheral blood cell counts to avoid interpreting peripheral neutrophilia or lymphocytosis as pancreatic inflammation.
Pancreatic Lesions
A summary of the World Health Organization scheme for histologic classification of pancreatic lesions of domestic animals is summarized in Box 19-2.9
Neoplasia
Adenoma
Benign exocrine epithelial tumors (i.e., exocrine adenomas, ductal [tubular] adenomas, or acinar adenomas) are rare in small animals and far less common than their malignant counterparts. They are generally small, solitary lesions found incidentally on imaging or necropsy examination. Histologically, they are partially or totally encapsulated, distinguishing them from the more common lesion of nodular hyperplasia.5,9,10 Cytologically, adenomas cannot be distinguished from normal or hyperplastic pancreatic tissue (see Figure 19-1). If abundant, uniform pancreatic exocrine epithelial cells are noted in concert with imaging findings suggestive of a solitary, solid lesion, differential diagnoses should include an adenoma, well-differentiated carcinoma, or a hyperplastic nodule (see Figure 19-1).
Adenocarcinoma
Malignant tumors of the exocrine pancreas (i.e., adenocarcinomas, ductal [tubular] adenocarcinomas, or exocrine carcinomas) are rare in the dog and cat with incidences of 17.8 in 100,000 patient years at risk in the dog and 12.6 in 100,000 patient years at risk in the cat.10,11 This is in contrast to human medicine in which pancreatic malignant tumors are the fifth leading cause of cancer-related death in the United States, with ductal adenocarcinomas accounting for > 90% of these malignancies.11 The histiogenesis of adenocarcinomas in small animals remains uncertain. One author suggests that a ductular origin is suggested based on tubular architecture, but ultrastructural analysis indicates that acinar cells may be the originator cell type,10 whereas other authors suggest that they can arise from either ductular or acinar epithelium, and often have features of both.5
In dogs, there is an increased incidence of pancreatic adenocarcinoma with aging, and Airedale terriers, boxers, Labrador Retrievers, and cocker spaniels may be at increased risk.11 In one study of 13 dogs and cats, the average age at diagnosis was 9 and 10 years of age for dogs and cats, respectively.12 Distant metastases are common at the time of diagnosis. In one study, 85% of the dogs and cats with pancreatic adenocarcinoma had distant metastases at the time of diagnosis, and 88% of the patients had metastatic disease at the time of necropsy.12 Common metastatic sites include abdominal or thoracic lymph nodes, mesentery, adjacent gastrointestinal (GI) organs (including liver, duodenum, and jejunum), lungs, and less frequently, spleen, kidney, and diaphragm.12 Local, destructive infiltration may destroy the common bile duct.
Clinical signs at the time of presentation are nonspecific but weight loss, vomiting, abdominal pain, and anorexia are common. Jaundice and cholestasis can result from obstruction of the bile duct by tumor and/or secondary liver disease. Clinicopathologic tests can show increases in pancreatic enzyme activity but evidence of extrahepatic biliary obstruction is more frequently seen, including elevations in alkaline phosphatase (ALP) and alanine aminotransferase (ALT) activitities. Neutrophilia is often noted.12 Described paraneoplastic syndromes include alopecia,13 exocrine pancreatic insufficiency,14 and cutaneous and visceral necrotizing panniculitis and steatitis.15
In dogs, pancreatic tumors frequently produce a mass, often in the midportion of the pancreas. In cats, tumors can be more diffuse and resemble nodular hyperplasia or chronic pancreatitis. Leakage of proteolytic enzymes from adenocarcinomas can be corrosive and may result in cystic change in the primary tumor and necrotizing steatitis in the omental and peritoneal fat.10 Histopathologically, pancreatic adenocarcinomas show a tremendous range of differentiation. Some are well-differentiated tubular adenocarcinomas that form acinar structures, whereas others may form more solid sheets of poorly differentiated cells that no longer resemble pancreatic acini. They may be associated with a dense supporting stroma with a resultant scirrhous reaction. Focal hemorrhage and necrosis can occur along with focal accumulations of inflammatory cells, including T-lymphocytes.10
Although pancreatic adenocarcinoma is far less common in dogs and cats than it is in people, similar to people, the majority of pancreatic neoplasms are malignant. Several studies of human pancreatic carcinoma have established objective cytologic criteria for the diagnosis of pancreatic carcinoma. The implementation of these criteria have resulted in a relatively high diagnostic sensitivity and specificity for the diagnosis of pancreatic adenocarcinoma ranging from 80% to 98% and of 93% to 100%, respectively.16 Similar standard criteria have not been developed in veterinary medicine. Given the increasing popularity of pancreatic FNB, objective cytologic criteria to diagnose pancreatic malignancy could be established for dogs and cats. Until that time, the criteria stated in human-based studies are compatible with important criteria of malignancy noted by the author.
Criteria of malignancy defining pancreatic adenocarcinoma are listed in Box 19-3. In the initial study, major criteria of malignancy included nuclear crowding and overlap (nuclei became oval, angular, and polygonal rather than round), nuclear membrane contour irregularities (grooving, notching), and irregular chromatin distribution (more applicable with alcohol-fixed specimens). Minor criteria included nuclear enlargement (or anisokaryosis, defined as a nucleus > 2.5 times the size of a red blood cell), single epithelial cells, necrosis, and mitosis.17 Later studies confirmed the utility of these initial criteria and suggested additional criteria including increased cellularity, anisocytosis, prominent or macronucleoli (large and irregular), cytoplasmic vacuolation, and coarsely clumped chromatin patterns. Mitoses, especially abnormal mitoses, favored malignancy.18,19 Combined, the strongest indicators of malignancy that define human pancreatic adenocarcinomas include anisokaryosis, loss of cellular cohesion (single cells are common), irregular nuclear contours, nuclear crowding, prominent nucleoli, and aberrant mitoses16–19 (see Box 19-3).