21 The manufacturers’ role in feed quality and safety
A discussion on methods used in feed manufacturing processes to assure feed hygiene and safety
Introduction
Quality in equine feedingstuffs
There are many harmful or undesirable substances that can enter the equine diet, for example:
• Microbiological contaminants
• Certain nutrients used at levels above those recognized as safe, for example selenium or rapidly digestible/fermentable carbohydrate.
Additionally to the list above, there is a further category of undesirable substance, those that are not considered harmful but are prohibited under the rules of racing and equestrian sport see Box 21.1 for recent examples of adverse feed product quality in the marketplace.
Box 21.1
Recent Examples of Adverse Feed Product Quality in the Marketplace
• April 2009: 21 polo ponies in Florida died of acute selenium toxicity, linked to a faulty manufacture of a supplement preparation (Anon 2009).
• May 2008: Nationwide product recall instigated after elevated Aflatoxin content found in equine and other feed products manufactured at three sites in the US (FDA 2008).
• Oct 2002 – Jan 2003: 43 horses, including 16 winners test positive for morphine in post race samples in UK and Ireland. The source was identified as racehorse feed (BHA 2004).
Characteristics of equine feeds
Whilst the equine diet is nominally described in terms of its forage and concentrate proportions, in practice a further degree of complexity exists due to the wide range of feed type, physical form, feeding rates, packaging and shelf life (Table 21-1) exhibited within the category of equine feedstuffs.
Table 21-1 Characteristics of Commonly Fed Equine Feedingstuffs with Typical Feeding Rates
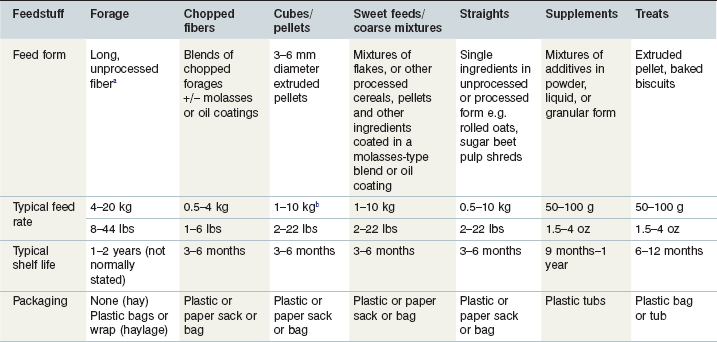
a In some countries, forage is presented in cube, pellet or wafer form.
b Where long forage is scarce or unavailable, complete pelleted fiber feeds, designed to provide the total daily diet, are available, with maximum feed rates therefore greater than the 10 kg described in the table.
Feed Ingredients
Whilst the main proportions of the equine diet are comprised of forage and cereal based ingredients, other components provide other essential nutrients (Table 21-2).
Table 21-2 The Nature and Source of Common Feed Ingredients Used in Equine Diets
| Ingredient source | Examples |
|---|---|
| Raw agricultural commodities | Oats, corn (maize), barley, peas, beans, lupins, naked oats, linseed, lucerne, straw, timothy hay, grass pellets, straw pellets |
| Human food by-products | Wheat bran, wheatfeed (wheat middlings), oatfeed (oat hull by-product), soy(a) hulls, rice bran, soy(a)bean meal, sunflower meal, linseed meal, sugar beet pulp, distillers grains, molasses. |
| Additives | Amino acids, vitamins, yeast products, mold inhibitors |
| Minerals | Limestone, calcium phosphates, salt, calcined magnesite (magnesium oxide) |
| Human grade ingredients | Vegetable oils (e.g. soya, corn), herbs |
Manufacturing facilities
Key Points –
The Manufacturer’s Quality Challenge
• To make products that consistently contain what they should contain and do not contain that which they should not
• This challenge applies across a complex and variable mix of feed forms, ingredients, feed rate, packaging and shelf life
• The consequences of not doing so are in the least, adverse publicity and loss of business for a company, but in the worst case can result in a serious health concern for the horse itself
Drivers of feed assurance
Regulatory requirements
• EU Regulation (EC) No 178/2002 lays down the general principles governing food and feed in general, and food and feed safety in particular, at EU and member state level. Of particular note are articles 15 and 18 of the Regulation. Article 15 states that feed shall not be placed on the market or fed to any food-producing animal if it unsafe. Article 18 states that traceability of food, food-producing animals, and any other substance intended or expected to be incorporated into a food or feed, shall be established at all stages of production, processing and distribution.
• The European Regulation on Feed Hygiene (Regulation EC 183/2005) lays down general rules on feed hygiene, conditions and arrangements ensuring traceability of feed, and conditions and arrangements for registration and approval of feed establishments, applicable to the whole feed chain. It explicitly states that “Feed business operators shall put in place, implement and maintain, a permanent written procedure or procedures based on the HACCP principles.” Industry guides to good practice were published in accordance with Article 22 of this Regulation, the European Feed Manufacturers Guide (2005) (http://www.fefac.org/code.aspx?EntryID=265, FEFAC) and the Feed Ingredients Standard (2007) (http://www.ifsa-info.net/lmbinaries/ifs.pdf. IFSA).
• EC regulation 1831/2003 on Feed Additives. Substances such as vitamins, trace elements and preservatives can only be used if they have undergone an assessment for safety, quality and efficacy.
• EC directive 2002/32 and Commission Regulation (EU) No. 744/2012 on Undesirable Substances and Products control contaminants such as heavy metals, certain mycotoxins, cyanogenic glycosides and dioxins. In addition, to listing maximum permitted levels for such substances, it prohibits the blending down of contaminated feeding stuffs (i.e., the mixing of a consignment in excess of a maximum permitted level for an undesirable substance, with another consignment of a lower contamination, to obtain a legal product).
In the US, the quality and safety of food for humans and animals is covered under one federal law, which is implemented by the Food and Drug Administration (FDA) and state agencies. Food for horses is considered to be “pet food”, but there is no legal distinction for food consumed by companion animals versus livestock. As in the EU there is no separate distinction in the regulation for animal dietary supplements (see Chapter 19).
Legislative and regulatory requirements concerning animal feed in the US are governed by the Federal Food, Drug and Cosmetic Act (FFDCA) – the overarching law that gives the FDA the authority to oversee the hygiene and safety of feed and feed ingredients (see www.fda.gov/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/default.htm).
The FFDCA makes animal feed manufacturers responsible for ensuring that feed products:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


