CHAPTER 9 The Liver
The microscopic examination of hepatic cytology is diagnostically rewarding when applied judiciously. Although fine-needle aspiration biopsy (FNAB) of the liver is practical and economical, it has defined (limited) diagnostic utility and its cavalier use can result in incomplete or inaccurate information. The information cytologically derived from a needle aspirate is often projected to equate with a histologic-based diagnosis (Roth, 2001). For inflammatory liver disease this is often an erroneous assumption since tissue architecture cannot be assessed and histopathologic findings cannot be quantified. These are critical elements for the microscopic assessment of the hepatic pathology (Ishak, 1994; Meyer, 1996). The ultrasonographic examination of the liver has added another dimension of potential “abnormal” findings that contribute to the complexities of working up patients with abnormal liver tests. It has facilitated the “guided” acquisition of cytologic specimens from focal lesions that can be either diagnostic (e.g., metastatic neoplasia) or descriptively nonspecific (e.g., vacuolar change) (Wang et al., 2004). The objective of this chapter is to suggest areas of hepatic pathology for which the cytologic interpretation of a FNAB can be diagnostic (Stockhaus et al., 2004) and to provide histologic comparators that illustrate the limitations of hepatic FNAB as well as provide differential considerations for the descriptive cytologic findings.
SAMPLING THE LIVER
Indications and Contraindications
A large cavitational lesion identified by ultrasound examination in an older dog, notably male German Shepherd Dogs and Golden Retrievers, is a relative contraindication for FNAB. The probability of a hemangiosarcoma is high, obtaining a definitive cytologic diagnosis is unlikely, and the potential of rupturing a necrotic capsule is a possibility. The spleen should be examined with ultrasound since hepatic metastasis is common. Exploratory surgery should be considered as both a diagnostic and treatment option.
Tumor seeding undoubtedly occurs but the frequency has not been determined (Evans et al., 1987; Navarro et al., 1998; Ishii et al., 1998). However, since treatment options are often limited and long-term survival usually has a poor prognosis, the use of FNAB as a diagnostic tool is a reasonable risk noting the aforementioned caveat pertaining to cavitational lesions.
Technique
For hepatomegaly, the site of needle entry into the abdominal cavity is at the “triangle” formed by the union of xiphoid and last left rib. Once the needle is within the hepatic parenchyma of the left lobe, it is directed in two or three different planes using a to-and-fro motion. Acquisition of the specimen can be obtained by either aspiration or by nonaspiration (“capillary” action) procedures (see Chapter 1). Generally, the nonaspiration approach should be tried first since it reduces the amount of blood contamination. If the specimen is nondiagnostic, the aspiration technique can be attempted. After collection of the specimen, the tip of the needle is placed over a glass slide and the contents expelled using a syringe filled with several cubic centimeters of air. Often multiple slide preparations can be made from a single sampling procedure by use of the compression method (see Chapter 1). The cytologic slide preparations are air-dried, fixed, and stained. Protection from formalin fumes is critical to preserve morphologic detail (no open formalin containers can be in the room prior to fixation and staining). At least one unfixed cytologic slide preparation should be saved for potential special stains such as a Gram, copper, iron, Congo red, or immunocytochemical stain.
Occasionally, the gallbladder or large bile duct is penetrated as evidenced by yellow to green to dark-green fluid in the syringe. Aspiration should be continued until no more fluid is obtained. As long as the biliary tissue is healthy, it will heal without side effects, although it is prudent to closely observe the patient for 24 hours for signs of peritonitis. Feeding a small amount of food or the oral administration of corn oil 30 minutes prior to FNAB has been suggested as means of contracting the gallbladder and reducing the risk of penetration. While there is probably no downside, the unpredictability of the canine gallbladder’s response to meal-stimulated contraction (Rothuzien et al., 1990) makes the procedure more reassuring for the operator than helpful to the patient in most cases. Light-yellow, acellular fluid also can be obtained from cystic lesions, which are especially prominent in cats. These would have been identified as cavitational lesions with ultrasonography and are probably congenital. Obtaining clear to whitish mucinous fluid from a cavitational lesion is suggestive of a cystadenoma.
NORMAL LIVER AND GALLBLADDER
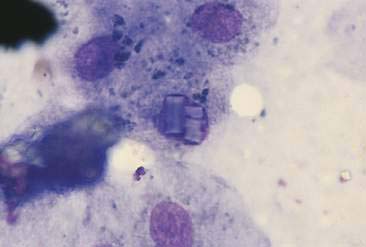
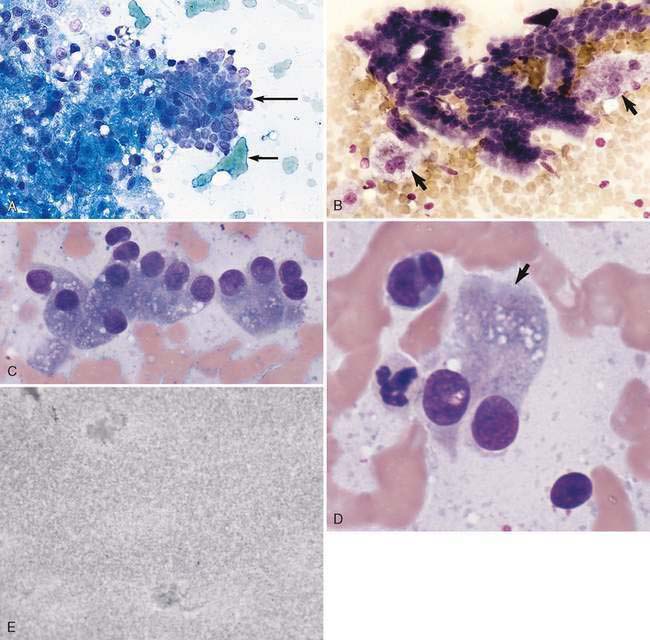
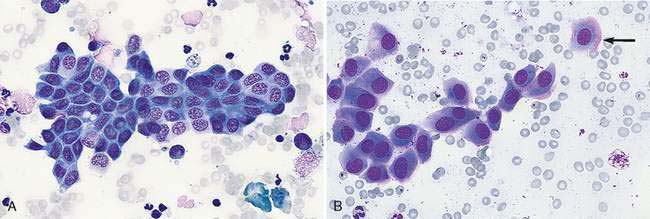
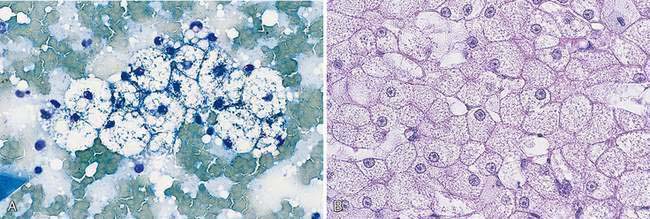
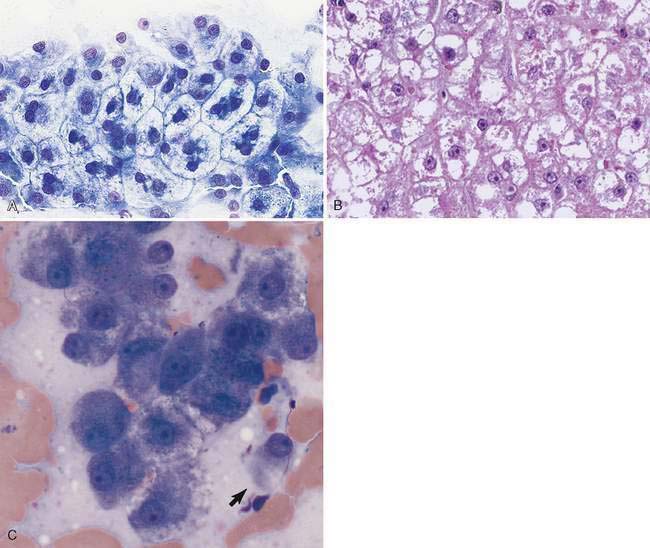
Hepatocytes constitute the predominant cell type of a normal liver (Fig. 9-1A&B). They are slightly oval to polygonal plump cells 25 to 30 μm in diameter (approximately 3 to 4 erythrocyte diameters). They contain a centrally placed, round to slightly oval nucleus composed of a coarse network of chromatin and a nucleolus that may be prominent. The nucleus is surrounded by abundant, moderately bluish to basophilic cytoplasm that is often granular appearing and punctuated by granular, pinkish hues because of the varying tinctorial properties of the different organelles. Hepatocytes appear to increase in size and number of nuclei per cell in older dogs (Stockhaus et al., 2002). An occasional mast cell is a normal finding within the hepatic parenchyma (Fig. 9-1C). Neutrophils are more frequent in young and older dogs than middle-aged dogs (Stockhaus et al., 2002). Rectangular nuclear crystalloid inclusions (Fig. 9-2) are noted in clinically healthy dogs of all ages with an unknown clinical significance. Dense clumps of biliary epithelial cells can be observed occasionally (Stockhaus et al., 2002). The dense collection of their basilar nuclei give the cells a cuboidal shape (Fig. 9-3A). Larger bile ducts including the common bile duct are lined by ciliated columnar cells (Fig. 9-3B-D). Bile fluid from the gallbladder appears dark green grossly but a shiny golden-, yellow as an unstained smear on a glass slide. With Romanowsky staining the fluid smear appears basophilic and microscopically displays a blue-gray finely granular appearance (Fig 9-3E) that is often referred to as “white bile.” Fluid from the gallbladder or common bile duct has been shown to be mucinous in the dog (Owens et al., 2003). Other cells observed during the sampling procedure of the liver are sheets of mesothelial cells that can be mistaken for biliary epithelium or a neoplastic cell population (Fig. 9-4A&B).
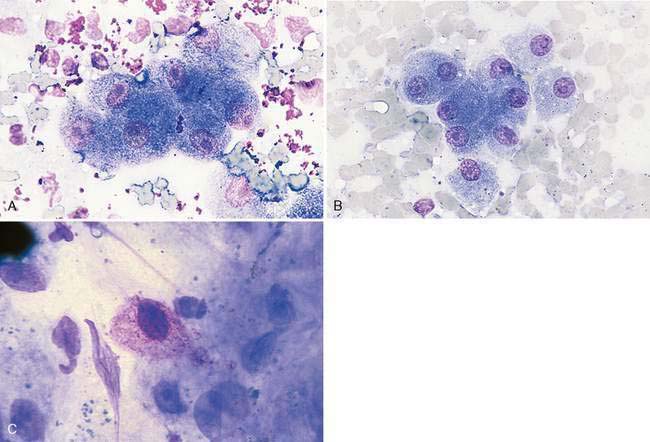
FIGURE 9-1 A-B, Normal hepatocytes. A, Clumps of greenish-stained erythrocytes and small, irregular clumps of metachromatic-stained gel used for ultrasound examination surround this island of hepatocytes. (Wright-Giemsa; HP oil.) B, An occasional binucleated hepatocyte (lower left of center) is a normal finding. (Wright-Giemsa; HP oil.) C, Hepatic mast cell. An occasional mast cell is a normal finding. The granules often do not stain intensively. (Wright-Giemsa; HP oil.) Contrast this image to an example of a hepatic mast cell neoplasia (Figures 9-30A&B).
NON-NEOPLASTIC DISEASES AND DISORDERS
Nuclear Changes
A nuclear inclusion that is more likely to be observed in chronic liver disease appears as a hollow, globule-like structure (Fig. 9-5A). It represents a membrane-bound cytoplasmic invagination that contains glycogen and mitochondria (Stalker and Hayes, 2007). This should not be mistaken for a viral inclusion. Canine adenovirus 1 infection produces viral inclusions (Fig. 9-5B&C) that appear as large, magenta, homogenous structures of various sizes in dogs with infectious canine hepatitis. There is associated hepatocellular necrosis and nuclear swelling causing a pale rim around the inclusion and chromatin margination producing the appearance of a thickened nuclear membrane.
Cytoplasmic Changes
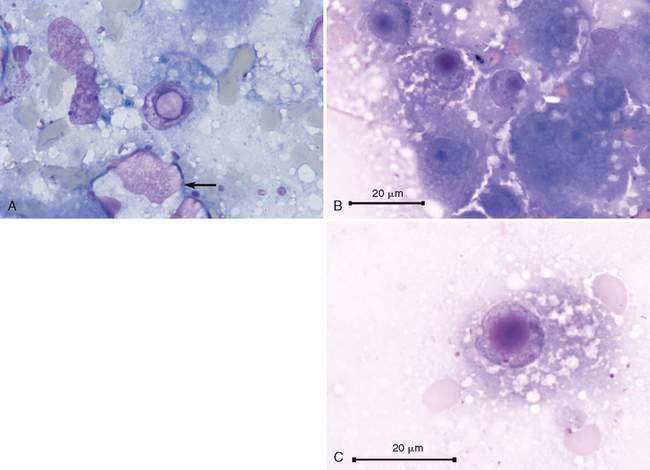
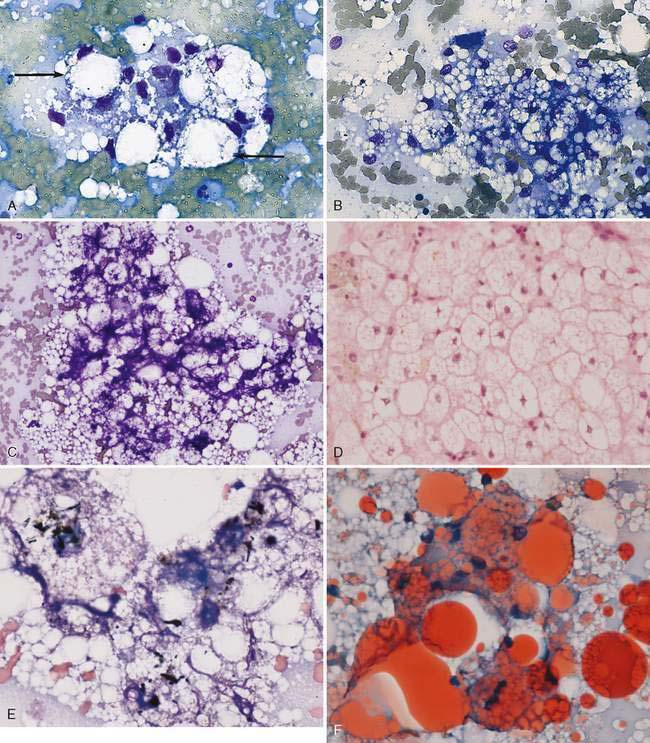
Hepatocellular cytoplasmic changes are common in association with metabolic disease and secondary to injury. Discrete small (microvesicular) or large (macrovesicular) vacuoles in the hepatocyte are representative of lipid that has been cleared during the staining process (Fig. 9-6A-E). Excess accumulation of lipid in the liver is referred to as lipidosis, fatty liver, or steatosis. The feline hepatic lipidosis syndrome is the most common disease associated with this cytoplasmic alteration. One drop of oil red O and one drop of new methylene blue placed together on an unfixed cytologic specimen along with a coverslip helps define the presence of lipid as the stain is readily taken up by lipoid substances (Fig. 9-6F). An underlying disease such as cancer and pancreatitis (identified with ultrasound examination and/or feline specific lipase) should be considered before classifying the lipidosis as idiopathic (Ferreri, 2003). Congenital lipid storage diseases are a cause of hepatomegaly in young animals due to diffuse hepatocyte vacuolar change (Brown et al., 1994) (Fig. 9-7A&B). Aspiration of mesenteric fat during the collection process is an artifact that can cause a potential misdiagnosis (Fig. 9-8).
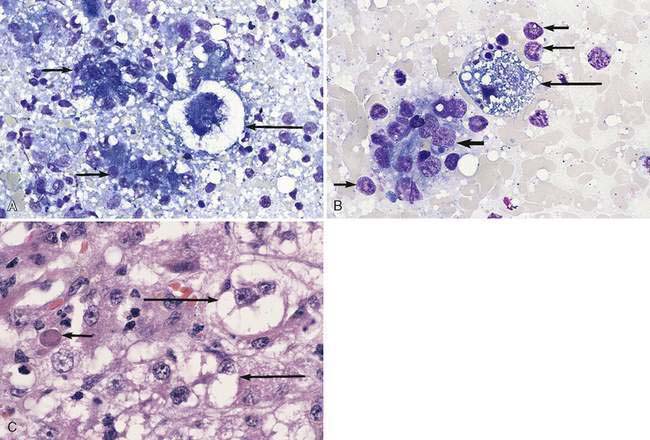
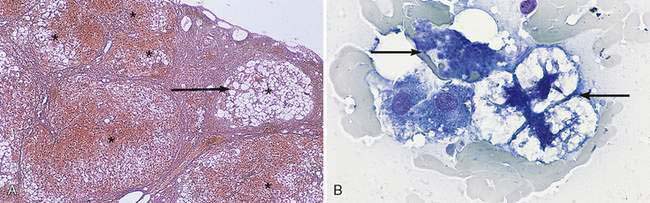
Hepatocellular cytoplasmic rarefaction (lesser cytoplasmic density than normal) that does not form discrete vacuoles can be caused by increased glycogen or water content and may occur in both dogs and cats. The latter type of histomorphologic change is also referred to as hydropic (ballooning) degeneration. The term vacuolation is often used as a nonspecific rubric for cytoplasmic rarefaction. The increased storage of glycogen that occurs in association with either hyperadrenocorticism causing hypercortisolemia or the administration of exogenous corticosteroids is a common cause of hepatocellular cytoplasmic rarefaction in the canine liver (Fig. 9-9A-C) and rarely in cats (Schaer and Ginn, 1999). The unique, robust nature of the canine hepatocyte to store glycogen in response to hypercortisolemia can be sufficiently dramatic to result in generalized hepatomegaly. The volume of the hepatocyte can be increased up to three-fold by excess glycogen accumulation (Kuhlenschmidt et al., 1991). Hepatocytes with excess glycogen are swollen, retain a centrally located nucleus, and lack discrete cytoplasmic vacuoles typically associated with lipid accumulation. A periodic acid-Schiff (PAS) staining method, with and without amylase (diastase) digestion, is used to distinguish cytoplasmic glycogen from mucin. Glycogen in the hepatocytes is removed by diastase treatment giving a negative reaction to PAS while neutral mucin will be resistant to digestion and thus remain PAS-positive. When hyperadrenocorticism and treatment with glucocorticoid medications have been eliminated as causes of the “vacuolar hepatopathy,” a search for an underlying disease is warranted (Sepesy et al., 2006). Various types of hepatocellular injury such as toxic insults and hypoxia will alter the integrity of the cell membrane and cytoplasmic organelles, resulting in increased cellular water content. As a consequence, cytoplasmic rarefaction (hydropic degeneration) is observed morphologically and cannot always be confidently distinguished from the cytoplasmic change associated with increased cytoplasmic glycogen (Fig. 9-10A–C). In aged dogs, hepatic nodular regenerative hyperplasia is common. The number of nodules ranges from a few to many, and they are often composed of hepatocytes with cytoplasmic rarefaction and/or vacuolation (Stalker and Hayes, 2007) (Fig. 9-11A&B).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree