CHAPTER 17 The skin is the largest organ in the body and has haired and hairless portions (Figs. 17-1 and 17-2). The skin consists of epidermis, dermis, subcutis, and adnexa (hair follicles and sebaceous, sweat, and other glands). The histologic structure varies greatly by anatomical site and among different species of animals. The haired skin is thickest over the dorsal aspect of the body and on the lateral aspect of the limbs and is thinnest on the ventral aspect of the body and the medial aspect of the thighs. Haired skin has a thinner epidermis, whereas nonhaired skin of the nose and pawpads has a thicker epidermis (see Figs. 17-1 and 17-2). The skin of large animals is generally thicker than the skin of small animals. The subcutis, consisting of lobules of adipose tissue and fascia, connects the more superficial layers (epidermis and dermis) with the underlying fascia and musculature. Fig. 17-1 Normal skin, haired, thorax, dog. Fig. 17-2 Normal skin, hairless, pawpad, dog. The epidermis is divided into layers based on the morphology of the keratinocyte, the major cell type of the epidermis. The epidermis of haired skin consists of four basic layers: stratum corneum, stratum granulosum, stratum spinosum, and stratum basale (Fig. 17-3). The epidermis of hairless skin consists of five layers; the fifth layer is the stratum lucidum, which is located between the stratum granulosum and stratum corneum. Keratinocytes originate from germinal cells in the stratum basale of the epidermis, ascend through the layers of the epidermis, changing in appearance and other characteristics in each layer until they reach the stratum corneum as fully keratinized, dead corneocytes. Keratinocytes are continuously shed from the stratum corneum. The transit time for a keratinocyte from the stratum basale to shed in the stratum corneum is approximately 1 month, although this time can be accelerated in some disorders such as primary seborrhea characterized clinically by scaling. Fig. 17-3 Schematic diagram of the structure of the skin. The epidermis and dermis are separated by a basement membrane. In hairless areas, such as the pawpads and nasal planum, this junction is irregular because of epidermal projections that interdigitate with dermal papillae (e.g., rete ridges/also known as rete pegs), thus strengthening the epidermal-dermal attachment by providing resistance to shearing. In densely haired areas, the junction is smoother and has an undulating appearance as the epidermal-dermal attachment is strengthened by the hair follicles. The more sparsely haired skin of pigs has more epidermal-dermal interdigitations (rete ridges) and fewer hair follicles. The basement membrane zone is composed of hemidesmosomes of basal cells (i.e., keratin intermediate filaments and attachment plaques), the lamina lucida (plasma membrane, subdesmosomal dense plate, and anchoring filaments), and the lamina densa (i.e., type IV collagen), which also serve to anchor the epidermis to dermis (Fig. 17-4). The importance of the basement membrane in anchoring function is noted in some immune-mediated diseases in which antibodies target, bind, and ultimately damage a component in the basement membrane and result in the formation of bullae (see the discussion on reactions characterized grossly by vesicles or bullae as the primary lesion and histologically by vesicles or bullae within the basement membrane [bullous dermatoses] section on Selected Autoimmune Reactions). The basement membrane zone also serves as a scaffold for migration of epidermal cells in wound healing and as an initial barrier to invasion of the dermis by neoplastic keratinocytes. Fig. 17-4 Schematic diagram of basement membrane structure of the skin. The skin is an important sensory organ containing millions of microscopic nerve endings that perceive itch, pain, temperature, pressure, and touch (Fig. 17-5). The nerve endings consist of Meissner’s corpuscles, pacinian corpuscles (Pacini’s corpuscles), free sensory nerve endings, and mucocutaneous end organs (similar to Meissner’s corpuscles but located in mucocutaneous skin). These nerve endings are minute, and the free sensory nerve endings are so delicate that they require special staining techniques, such as silver impregnation, to be visualized microscopically. The sensations of itch, pain, touch, temperature, and displacement of body hair are detected by the free sensory nerve endings. Itching, a form of mild pain that promotes the desire to scratch, is one of the most common reasons animals are presented to veterinarians. The sensations of touch and pressure are detected by Meissner’s and Pacini’s corpuscles. Sensations detected by free sensory nerve endings and by the corpuscles are transmitted to the spinal cord via the dorsal root ganglia. Sensory fibers to the facial area are supplied by the trigeminal nerve. Motor fibers (adrenergic and cholinergic) are supplied by the sympathetic component of the autonomic nervous system (see Fig. 17-5). Adrenergic fibers travel from the spinal cord through postganglionic fibers in peripheral nerves and arborize into plexuses that innervate blood vessels, arrector pili muscles, and apocrine sweat glands. Stimulation by these adrenergic fibers causes vasoconstriction and piloerection (raising of the hair shafts). Cholinergic fibers travel from the spinal cord and arborize into plexuses that innervate the eccrine sweat glands. Stimulation of these fibers in humans causes widespread eccrine sweating, important in thermoregulation and recognized clinically as “beads of sweat” on the skin. Because the eccrine ducts open directly on the surface of the skin, the secretion is more easily seen clinically. This phenomenon does not occur in dogs or cats because they lack eccrine glands in haired skin. However, cholinergic and to a lesser degree adrenergic fiber stimulation in dogs and cats causes sweating of the eccrine glands of pawpads at times of excitement or agitation. In the haired skin, equine sweat glands are considered to be the epitrichial (apocrine) type, where the duct opens into the follicular canal near the skin surface, but less commonly the duct may open in a depression near the follicle opening or directly on the skin surface. As in humans, sweating in the horse is important in thermoregulation. However, the precise mechanisms that control sweating in the horse are unknown. The horse has a rich supply of vessels and nerves around sweat glands. It appears that equine sweat gland secretion is controlled by an interaction among neural, humoral, and paracrine factors. The only other domestic animal in which apocrine gland secretion is thought to play a thermoregulatory role is cattle, but sweating is not typically clinically visible except in horses. Fig. 17-5 Schematic diagram of cutaneous innervation. The growth of hair occurs within hair follicles in a sequence of stages (Fig. 17-6). These stages include hair genesis, growth, maturation, and loss. In the anagen stage of the hair cycle, mitotic activity and growth occur. The catagen stage is a transitional phase during which cellular proliferation ceases. The follicle then enters a resting stage, telogen, after which mitotic activity and new hair production resumes. The exogen stage is the phase in which the old hair is shed. In many animals hair follicle growth occurs in cycles, resulting in periodic loss or shedding of the hair coat. The reason the hair growth occurs in cycles is not clear. Some hypotheses include that the cycle provides the ability to: (1) shed fur to cleanse the body surface, (2) adapt and change body cover in response to changing environment (winter to summer) or social conditions, or (3) protect against malignant transformation that might occur in a rapidly dividing tissue. The regulation of hair cycling is exceedingly complex and incompletely understood. Factors that play a role include genetics, photoperiod, temperature, nutrition, hormones, health status, and neural mechanisms. Genetic factors determine the hair shaft length (e.g., short-haired versus long-haired breeds of dogs). The exact signals that control the hair cycle have not been identified; however, growth factors, such as epidermal growth factor, fibroblast growth factor (FGF), hepatocyte growth factor, platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and insulin-like growth factor (ILGF), have been localized to the skin and hair follicles. These growth factors probably play a crucial role in regulation of the hair cycle and follicle growth. Fig. 17-6 Schematic diagram of the hair cycle. Forms of hair follicles vary in different animals (Fig. 17-7). Horses and cattle have evenly distributed simple follicles with one large (i.e., primary) follicle, usually with sebaceous and apocrine glands and arrector pili muscles. Pigs have simple follicles grouped in clusters. Goats, dogs, and cats have compound follicles that consist of primary follicles and smaller secondary follicles. Sheep have simple follicles in hair-growing areas and compound follicles in wool-growing areas. Primary follicles have the hair bulb rooted more deeply in the dermis than secondary follicles. The depth of the hair bulbs varies with species. In dogs and cats, the anagen hair bulbs of primary follicles are at the dermal-subcutaneous junction, whereas in horses and cattle the anagen hair bulbs are in the mid-dermis. In all species, the bases of telogen follicles are more superficially located than the bases of anagen follicles. Typically, primary and secondary hair shafts emerge through a common follicular opening. Tactile hairs include sinus and tylotrich hairs. Sinus hairs, also termed vibrissae, arise in simple follicles with a blood-filled sinus located between the inner and outer layers of the dermal sheath. Sinus hairs generally occur on the nose, above the eyes, on the lips and throat, and on the palmar aspect of the carpus of cats. Sinus hairs function as mechanoreceptors (i.e., touch receptors). Tylotrich hairs also function as mechanoreceptors and are scattered among the regular body hairs. The arrector pili muscles extend from the connective tissue sheath of the hair follicles at the junction of the middle and inferior portion of the follicle and attach to the superficial dermis. The arrector pili smooth muscles are oriented almost perpendicularly to the wall of the follicle and are well developed on the back of animals, especially dogs. Muscle contraction causes erection of hairs and expression of the contents of sebaceous glands. Fig. 17-7 Schematic diagram of the skin and simple and compound hair follicles. There are two basic types of sweat glands: apocrine glands and eccrine glands. Apocrine glands are located throughout haired areas of skin in domestic animals and are tubular- or saccular-coiled glands (see Fig. 17-7). The ducts of the apocrine glands open in the superficial portion of the hair follicle; thus these glands are also called epitrichial glands. The glands are lined by secretory cuboidal to low columnar epithelium surrounded by contractile myoepithelial cells. Other apocrine glands include the interdigital glands of small ruminants, glands of the external ear canal and eyelids of domestic animals, anal sac glands of dogs and cats, and the mental organ of pigs. Eccrine glands are merocrine in secretion and in contrast to ducts of apocrine glands, the ducts open directly onto the surface of the epidermis. Thus eccrine glands are also called atrichial glands. They are tubular glands lined by cuboidal epithelium surrounded by myoepithelium and are confined mainly to pawpads of dogs and cats, frog region of ungulates, carpus of pigs, and nasolabial region of ruminants and pigs. The digital pads of dogs and cats have a thick epidermis composed of all layers, including the stratum lucidum. The surface is covered by compacted layers of stratum corneum and is smooth in the cat; however, in the dog, the surface is covered by conical papillae that conform to the outline of the epidermal surface (see Fig. 17-2). The epidermis and dermis interdigitate via rete ridges and dermal papillae, thus providing resistance to shear forces. Eccrine (atrichial) glands are present in the dermis and the adipose tissue. Lobules of adipose tissue that act as a cushion are subdivided by collagenous stroma and elastic tissue. The skin is not only the largest organ in the body, but one of the most important. Without the skin, terrestrial mammalian life could not exist. The skin has numerous functions, which are listed in Box 17-1. The skin prevents significant loss of fluid and electrolytes (e.g., the stratum corneum barrier), protects against physical and chemical injury (e.g., the stratum corneum barrier, keratin filaments, desmosomal and hemidesmosomal junctions, collagen, and elastic fibers), participates in temperature and blood pressure regulation (e.g., the hair coat, sweat glands, and vascular supply), produces vitamin D (e.g., ultraviolet [UV] light photolysis of dehydrocholesterol), serves as a sensory organ (e.g., tactile hairs, Merkel cells, and nerves), and stores fat, water, vitamins, carbohydrates, protein, and other nutrients (e.g., subcutaneous fat). Absorption, although not a primary function, also occurs. In addition, the keratinocyte, a major source of cytokines and antimicrobial peptides, is now considered to be an integral part of the innate and adaptive immune systems protecting against microbial injury and participating in inflammation and tissue repair. The route by which an infectious agent gains entry into the body is called the portal of entry (Box 17-2). Many pathogens can only cause disease when entering the body via their specific portal of entry. A few pathogens, such as hookworm larvae, are able to penetrate intact normally functioning skin. Dermatophytes are able to colonize the cornified structures (hair, claws) and the stratum corneum and cause disease without ever entering living tissue. Clinical disease in a dermatophyte infection is the result of the host’s reaction to the organism and its by-products. The skin only becomes an efficient portal of entry for microorganisms when the barrier is damaged by trauma, excessive moisture, heat or cold, or by disruption of the normal flora of the integument. A number of microorganisms (e.g., Staphylococcus intermedius, Streptococcus sp., Corynebacterium pseudotuberculosis, Pasteurella sp., Proteus sp., Pseudomonas sp., and Escherichia coli) gain entrance to the body by either entering through natural pores, such as hair follicles or glands with ducts that traverse the epidermis, or by the parenteral route, which includes all types of breaks in the skin, including injections, insect bites, and other types of wounds. Organisms that are able to inhabit hair follicles, such as mites or bacteria, gain entry to the body when the wall of the follicle is ruptured, leading to emptying of follicular contents into the dermis. Similarly, rupture of glands or ducts can lead to entry of microorganisms. From here, infectious agents can stimulate a robust host immune response or possibly spread to other areas of the body by gaining entry to the bloodstream or traveling to regional and distant lymph nodes via lymph flow. Intact skin with its waterproof barrier provides some protection against weak acids and alkali substances and water-soluble compounds, but certain lipid-soluble compounds can be absorbed directly through intact skin as can some artificially engineered gases developed for chemical warfare. UV radiation (UVR) can damage the skin by direct exposure if the body’s natural defenses, such as the hair coat and melanin pigments, are not present or are inadequate. The lesions of solar (actinic) dermatitis (see the section on Disorders of Physical, Radiation, or Chemical Injury: Solar (Actinic) Dermatosis, Keratosis, and Neoplasia) typify the effects of chronic exposure to UVR. In addition to solar dermatitis, squamous cell carcinomas, hemangiomas, and hemangiosarcomas have an increased tendency to develop in skin chronically damaged by UVR. Barriers of the skin against physical injury are listed in Box 17-3. The hair coat, particularly the long dense hair coat of some dogs and cats, serves as a physical barrier to temperature extremes, UVR, and minor trauma. The hair coat also sheds water as a result of the lipids provided by sebaceous gland secretion. Vibrissae, or tactile hairs, and sensory neurons provide awareness of the physical environment, allowing the animal to make appropriate reactions for survival such as reflex responses to heat and other noxious stimuli. Claws, especially on cats, serve as a quite effective barrier against predators by providing traction for climbing and serve as weapons to be used against aggressors. Horns of cattle, sheep, and goats also provide some physical defense capabilities. Anatomic features of the skin that provide resistance to physical injury are listed in Box 17-3. Hair follicles help anchor the epidermis to the dermis, as do epidermal-dermal interdigitations, thus these interdigitations are most numerous in nasal planum and pawpad where hair follicles are absent and resistance to shearing force is necessary. Host defense against mechanical injury is also provided by the tightly bundled keratin filaments of the corneocyte, the resilience of the cornified envelope, the adhesion of the cornified envelope and intercellular lipids, and the corneodesmosomes. In addition, the keratinocytes contain keratin filaments and form desmosomal junctions with adjacent cells (see Fig. 17-4). The keratin filaments perform a structural role (i.e., cytoskeletal) in the cells, and the desmosomes promote adhesion of epidermal cells and resistance to mechanical stresses. The basement membrane anchors the epidermis to the dermis via hemidesmosomes providing structural integrity against trauma. Dermal collagen and elastic tissue provide resilience and strength to the skin and support for the vessels, nerves, and adnexa. The panniculus protects against surface trauma by providing some shock absorption (e.g., pawpads), by facilitating movement, and by anchoring the dermis to fascia. Thus the various components of the epidermis, dermis, adnexa, and panniculus provide a flexible interconnecting framework to protect the host against mechanical injury. Innate immunity is a primitive, highly conserved response that quickly detects and impairs pathogens and harmful environmental stimuli encountered daily in life and does not require antigen-specific receptors. Innate immunity protects the host during the first 7 days of exposure to a pathogen before development of an adaptive immune response, and also initiates and assists the adaptive immune response (see Web Table 17-1). The diversity of microorganisms requires equal diversity in host defense responses. The first phase of innate host defense consists of the barrier of the stratum corneum, which prevents pathogen adherence and provides an antimicrobial surface consisting of antimicrobial peptides and fatty acids. The antimicrobial peptides (e.g., β-defensins and cathelicidins) are effective against many organisms, including viruses, bacteria, protozoa, and insects, and probably kill some of these pathogens by damaging their lipid membranes. The surface barrier also includes normal flora of nonpathogenic bacteria that competes with pathogenic microorganisms for nutrients and for attachment sites on cells. The normal flora also produces antimicrobial substances that prevent pathogen colonization. Because the dry cornified surface is such an effective barrier to pathogens, a wound or abrasion is usually necessary for a pathogen to gain entrance. Web Fig. 17-1 provides an illustration of how the innate and adaptive (acquired) immune systems participate in host defense against bacterial pathogens that have gained entrance into the skin through a wound in the epidermis. When injured, keratinocytes release a variety of antimicrobial peptides, chemokines, and cytokines, which activate endothelial cells and attract macrophages, neutrophils, and lymphocytes to the site of injury. Early key cells that play a role in innate immunity are the tissue macrophages that contact, bind, phagocytose, and thereby eliminate many types of pathogens. Pathogen recognition is mediated by pattern-recognition receptors (PRRs), including Toll-like receptors and others, that recognize repeating patterns of molecular structures common to broad classes of pathogens and efficiently differentiate pathogen antigens from self-antigens. The repeating patterns of molecular structures on pathogens are called pathogen-associated molecular patterns (PAMPs). WEB TABLE 17-1 Innate Immunity Host Defense Mechanisms Web Fig. 17-1 Diagram of interactions between the innate and acquired immune systems in response to bacterial infection of the skin. Whereas innate immunity works immediately to detect and destroy microorganisms, acquired or adaptive immunity develops later because the lymphocytes that contribute to adaptive immunity specific for the invading pathogen must increase in number by clonal expansion (see Web Table 17-2). The major components of the cutaneous adaptive immune system include keratinocytes, dendritic antigen-presenting cells (Langerhans’ cells and dermal dendritic cells), lymphocytes, and endothelial cells (see Web Fig. 17-1). WEB TABLE 17-2 Adaptive Immunity Host Defense Mechanisms Lymphocytes recognize pathogens (i.e., antigens) via cell surface receptors. The B lymphocytes have immunoglobulin molecules as the receptors for antigen, and on activation, B lymphocytes secrete immunoglobulin, which provides defense against pathogens (often bacteria) in the extracellular spaces. Antibody facilitates pathogen neutralization, complement activation, and enhanced endocytosis by phagocytes. In contrast, T lymphocytes have receptors that recognize foreign antigens expressed as peptide fragments bound to major histocompatibility complex (MHC) proteins (see Chapter 5 for a review). One class of T lymphocyte expresses the CD8 molecule on the surface (i.e., CD8+ T lymphocytes). These CD8+ T lymphocytes recognize peptide fragments bound to MHC I, then kill the cell, and thus are also called cytotoxic T lymphocytes. Atopic dermatitis (atopy) serves as an example of a common disease associated with impaired function of the epidermal barrier and immunity. It is a multifactorial, chronic and relapsing, often severely pruritic skin disease that affects humans, horses, dogs, and cats. It can cause severe discomfort including sleeplessness from pruritus, and is associated with secondary skin infections. Although the etiopathogenesis of atopic dermatitis has been studied most extensively in humans, many similarities with the human disease have been identified in atopic dogs. Advances in the knowledge of canine atopic dermatitis have occurred through the recent development and validation of animal models of the disease; these models have allowed more in-depth investigative studies in dogs and more precise comparisons of the disease between humans and dogs. Atopic dermatitis is a common problem in dogs; it is estimated to affect 10% to 15% of the population (Web Fig. 17-2). It is also a common human disease estimated to affect 5% to 20% of children. Similarities in the disease in humans and dogs include young age of onset; genetic inheritance; similar clinical lesion distribution; similar histopathologic lesions, including infiltration of immunoglobulin E (IgE+) CD1c+ dendritic cells; dry skin with increased transepidermal water loss; decreased stratum corneum ceramides (lipids); decreased epidermal filaggrin; increased colonization of surface staphylococci; positive atopy patch test; increased IgE-specific responses; and TH2-dominated immune responses. A major difference in the disease is that children with atopic dermatitis often develop asthma and allergic rhinitis, whereas dogs do not; the reason for this difference is currently unknown. Web Fig. 17-2 Atopic dermatitis, skin, dog.
The Integument
Structure
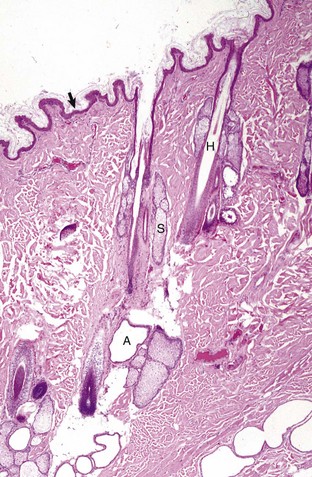
The epidermis (arrow) in haired skin has an undulating surface but lacks rete ridges. The epidermis in haired skin has fewer nucleated cell layers than the epidermis in nonhaired (hairless) skin such as that on the nose and pawpads (see Fig. 17-2); thus it is referred to as “thin” skin. Hair follicles (H), apocrine glands (A), and sebaceous glands (S) are present. The haired skin is thickest over the dorsal aspect of the body and on the lateral aspect of the limbs, and it is thinnest on the ventral aspect of the body and the medial aspect of the thighs. H&E stain. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
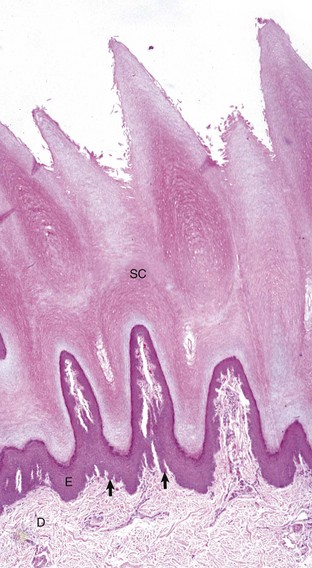
The epidermis (E) in hairless (nonhaired) skin has more numerous nucleated cell layers and more abundant stratum corneum than the epidermis in haired skin; thus it is referred to as “thick” skin. Note the dense zone of compact stratum corneum (SC) over the surface. The epidermis and dermal papillae in the superficial dermis (D) interdigitate to form rete ridges (arrows). The rete ridges strengthen the attachment between the epidermis and dermis. In the dog, the contour of the epidermal surface follows the epidermal ridges and thus is papillated. H&E stain. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Epidermis
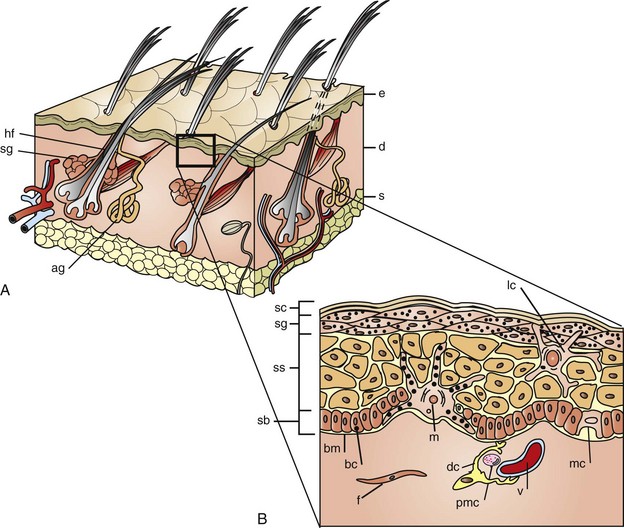
A, The skin is composed of epidermis (e), dermis (d), and subcutis (s), with adnexa consisting of hair follicles (hf), sebaceous glands (sg), and apocrine glands (ag). B, This projection of the epidermis demonstrates the progressive upward maturation of basal cells (bc) in the stratum basale (sb) through the stratum spinosum (ss), stratum granulosum (sg) into the cornified squamous epithelial cells of the stratum corneum (sc). Melanocytes (m), midepidermal dendritic Langerhans’ cells (lc), and Merkel cells (mc) are also present. The subjacent dermis contains small vessels (v), fibroblasts (f), perivascular mast cells (pmc), and dendrocytes (dc), potentially important in dermal immunity and repair. (Adapted from Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders; Gawkrodger DJ: Dermatology: an illustrated colour text, ed 2, New York, 1997, Churchill Livingstone.)
Basement Membrane Zone
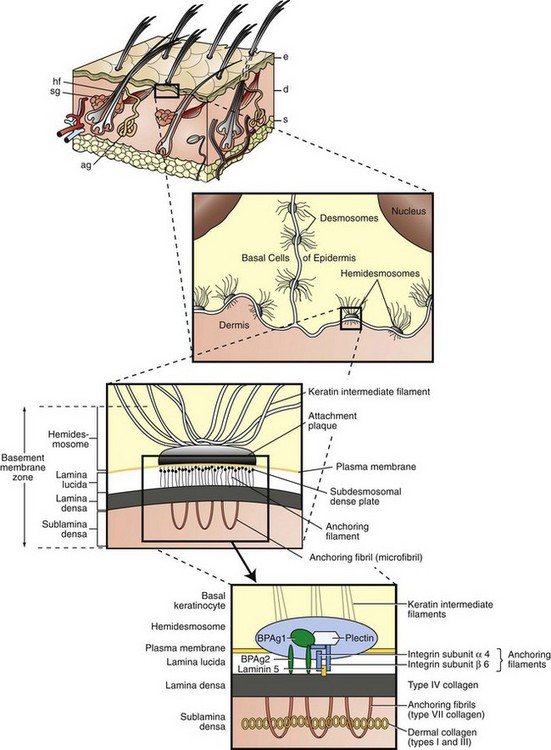
Keratinocytes attach to each other via desmosomes and to the basement membrane via hemidesmosomes. The projection of the basement membrane zone illustrates the multiple, interconnecting layers of this zone. The most superficial layer consists of the basal layer hemidesmosomes (keratin intermediate filaments and attachment plaques). The next layer, the lamina lucida, is an electron-lucent zone composed of the plasma membrane, subdesmosomal dense plate, and anchoring filaments. The deepest layer is the lamina densa, an electron-dense zone that consists of type IV collagen. Anchoring fibrils (type VII collagen), serve to attach the lamina densa and epidermis to the papillary dermis. The interconnecting layers of the basement membrane zone provide an important function in epidermal-dermal adherence, are the site of immune reactant deposition in cutaneous disease (see Table 17-11), and serve as a barrier to invasion by malignant epidermal tumors. ag, Apocrine glands; d, dermis; e, epidermis; hf, hair follicles; s, subcutis; sg, sebaceous glands. (Adapted from Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders; Rubin E, Farber JL: Pathology, ed 3, Philadelphia, 1999, Lippincott-Raven; and Elder DE: Lever’s histopathology of the skin, ed 10, Philadelphia, 2009, Lippincott Williams & Wilkins.)
Vessels and Nerves
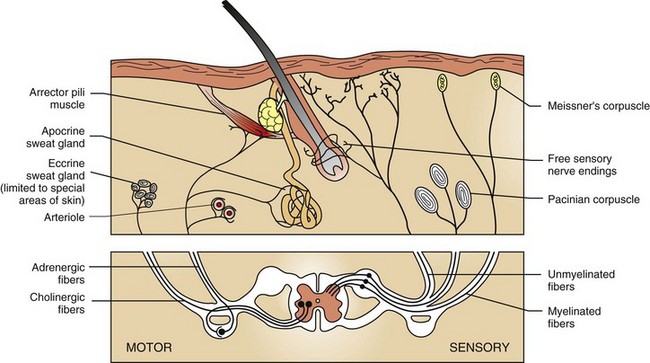
Cutaneous nerve endings transmit sensations of touch, pressure, temperature, pain, and itch (pruritus) via dorsal root ganglia to the central nervous system. Motor fibers in skin are supplied by the autonomic nervous system. Adrenergic fibers activate arterioles, arrector pili muscles, and apocrine glands; cholinergic fibers stimulate eccrine glands. (Adapted from Ackerman AB: Histologic diagnosis of inflammatory skin diseases, Philadelphia, 1978, Lea and Febiger.)
Adnexa
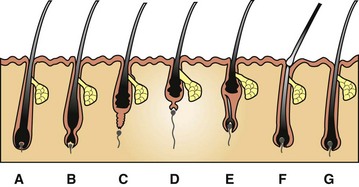
A, Anagen. During this growing stage, hair is produced by mitosis in epithelial cells covering the apex of the follicular papilla (dermal papilla), which is enveloped by hair matrix cells at the hair bulb. B, Early catagen. In this transitional stage, a constriction occurs at the hair bulb, and the hair shaft above this becomes a “club hair.” C, Catagen. The distal follicle becomes thick and corrugated and pushes the hair outward. D, Telogen. This is the resting stage in which the follicular papilla separates and an epithelial strand shortens to form a secondary germ. E, Early anagen. The secondary germ grows down to enclose the follicular papilla, and a new hair bulb forms. F, Exogen. This refers to the shedding of the old hair shaft. G, Anagen. The hair elongates as growth continues. Note that the anagen hair bulbs are located deeper in the dermis than the catagen and telogen hair bulbs. (Adapted from Scott DW, Miller WH Jr, Griffin CE: Muller and Kirk’s small animal dermatology, ed 6, Philadelphia, 2001, Saunders.)
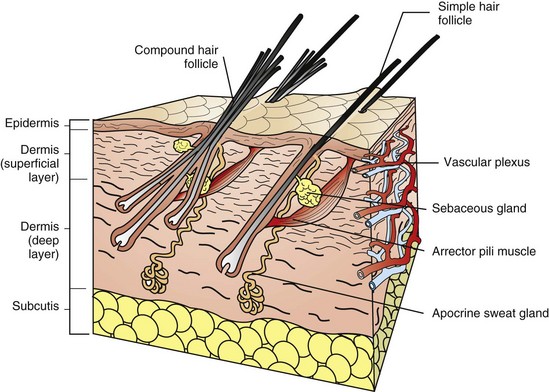
For the purpose of simplification, the vascular supply is illustrated on one face only. Note the simple hair follicle (right) and the compound hair follicle (left). Simple follicles consist of one large primary follicle with the hair bulb in the dermis or subcutis (depth varies with species), and with sebaceous and apocrine glands, and arrector pili muscles. Compound follicles consist of a large primary follicle and smaller secondary follicles. The hair bulbs of secondary follicles are located more superficially in the dermis than the hair bulbs of primary follicles. Secondary follicles may have sebaceous glands but lack apocrine glands and arrector pili muscles. (From Dellman DH, Brown EM: Textbook of veterinary histology, ed 3, Philadelphia, 1987, Lea and Febiger.)
Sweat Glands
Specialized Structures
Function
Portals of Entry
Defense Mechanisms
Physical Defense Mechanisms
Resistance to Mechanical Forces
Immunologic Defense Mechanisms
Stratum corneum barrier
Prevents pathogen adherence and provides antimicrobial surface
Macrophages (dendritic cells) with pattern-recognition receptors
Recognize broad classes of pathogens, differentiate pathogen antigens from self antigens, initiate Toll signaling pathway, and secrete cytokines, facilitating inflammation and innate immunity.
Toll signaling pathway
Promotes expression of large numbers of genes, resulting in production of cytokines, chemokines, and adhesion molecules important in inflammation and innate immunity.
Macrophages and neutrophils
Recognize, ingest, and destroy pathogens.
Endothelial cells
Express adhesion molecules and trigger kinin and coagulation systems, facilitating influx of plasma proteins and migration of leukocytes (cell trafficking) necessary to control infection.
Coagulation system
Forms blood clot in case of injury to control blood loss and prevents microorganisms from entering the bloodstream.
Complement enzyme cascade
Recruits inflammatory cells, opsonizes pathogens, and kills some pathogens.
Lipid mediators
Increase vascular permeability and induce influx and activation of leukocytes sustaining inflammatory responses.
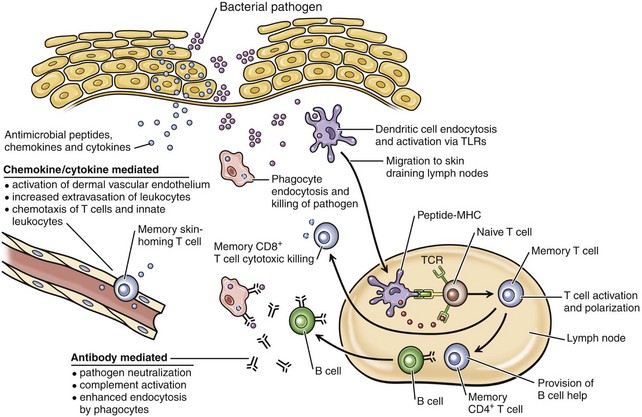
In response to bacteria that have breached the epithelial barrier, keratinocytes synthesize anti-microbial peptides, chemokines, and cytokines. These factors lead to activation of the dermal capillary endothelium, inducing the migration of innate leukocytes and memory T lymphocytes into the skin and additionally guiding these cells via chemotactic gradients. These factors and bacterial antigens activate innate phagocytes to kill ingested organisms and activate dendritic cells to migrate to the local skin lymph nodes. In the lymph nodes, dendritic cells present bacterial antigens to naive and central memory T lymphocytes, leading to stimulation of pathogen-specific lymphocytes. Effector CD8+ T lymphocytes exit the lymph node, home to inflamed skin, and kill pathogens. Helper CD4+ T lymphocytes provide help to B lymphocytes, inducing the production of antibodies that directly neutralize pathogens and lead to additional targeting of innate responses. Antibody-directed phagocytosis by innate cells leads to enhanced antigen presentation, further enhancing acquired responses. (From Clark R, Kupper T: J Invest Dermatol 125:629-637, 2005.)
Acquired (Adaptive) Immunity
Langerhans’ cells in epidermis and dendritic cells in dermis
Ingest and process antigen, present antigen to naïve T lymphocytes in lymph nodes, present antigen to sensitized T lymphocytes at site of injury, and produce cytokines that upregulate inflammation and immune responses.
T lymphocytes
After stimulation by antigen-presenting cells in lymph node, migrate back to the site of injury.
CD8+ (cytotoxic lymphocytes)
Recognize antigen expressed on the cell surface and kill the cell (cytotoxic lymphocytes); responsible for killing neoplastic cells, some bacteria and parasites, and all viruses that replicate inside cells.
CD4+ TH1
Activate macrophages, helping to control infection by intracellular bacteria.
CD4+ TH2
Activate B lymphocytes, helping eliminate extracellular pathogens.
B lymphocytes
Secrete immunoglobulin, providing defense against pathogens (often bacteria) in extracellular spaces.
Endothelial cells
Express adhesion molecules and bind to stimulated T lymphocytes.
Keratinocytes
Produce cytokines and growth factors upregulating or downregulating inflammation and immune responses.
Cytokines, chemokines, and adhesion molecules
Contribute as in innate immune response.
Disease Example of Barrier Dysfunction
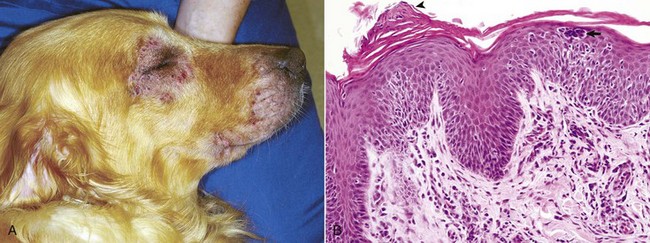
A, This Golden retriever has erythema, alopecia, and erosions in the skin around the eye and muzzle. The lesions are caused by self-trauma from rubbing and scratching as a result of pruritus. B, Photomicrograph from experimentally induced lesion of atopic dermatitis in the skin of a dog. The epidermis has acanthosis, mild spongiosis, and a few lymphocytes and clustered Langerhans’ cells (arrow). Focal parakeratosis (arrowhead) is also noted. H&E stain. (A courtesy Dr. David Duclos, Animal Skin and Allergy Clinic. B courtesy Dr. Thierry Olivry, College of Veterinary Medicine, North Carolina State University.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Integument
