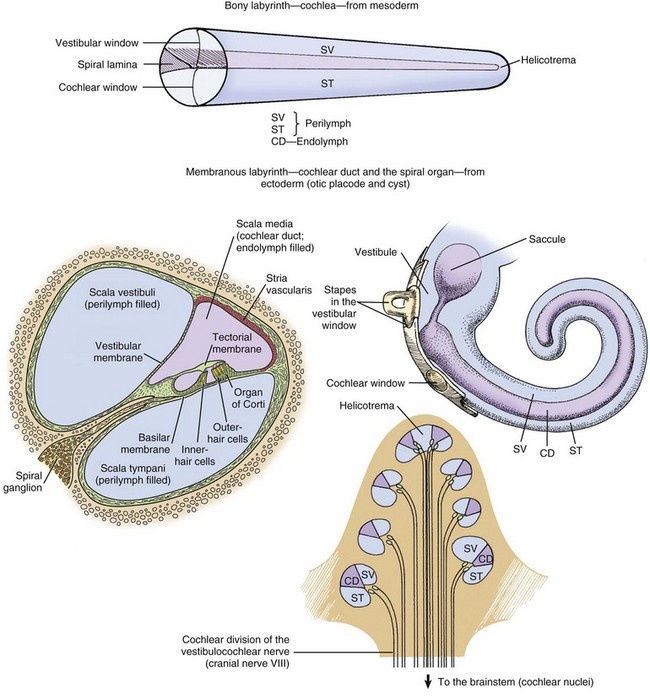
CHAPTER 20 The auricle functions to collect, focus, and direct sound down the funnel-shaped external acoustic meatus to the tympanic membrane (Fig. 20-1). Because the rostroauricular and caudoauricular muscles are innervated by motor branches of the facial nerve, the flexible (cartilaginous) auricle is able to move rostrally, laterally, and caudally about a central auricular axis. The position of the auricle can signal an animal’s behavior or emotion. Fearful cats often flatten their ears in a defensive posture, whereas angry horses often completely flatten their ears caudally as an initial warning before they strike. Alternatively, a dog may flatten its ears when content or when it is being verbally scolded. Fig. 20-1 Major regions of the ear. The external acoustic meatus (ear canal) is a conical opening made up of elastic cartilage and bone (Fig. 20-2). The more lateral portions are composed of auricular cartilage that narrows and overlaps with annular cartilage. Dense fibrous connective tissue forms a bridge between the annular cartilage ring and the osseus portion of the external acoustic meatus. In cats and dogs, the osseous portion (1) is a very narrow rim of bone and (2) is a broad opening exposing the tympanic membrane that is readily visible during otic examination. In horses, ruminants, and pigs, the osseous portion of the external acoustic meatus is an elongate cylinder of bone with a narrow lumen (Fig. 20-3). In horses, the junction between the cartilaginous and osseous portions of the external acoustic meatus is grossly identified by an abrupt change from pigmented to nonpigmented epithelium. Visualization of the deeper portions of the external acoustic meatus in livestock species requires specialized equipment and heavy sedation. Fig. 20-2 External acoustic meatus, osseous and cartilaginous portions, cross-section through the left ear, dog. Fig. 20-3 Osseous external acoustic meatus, macerated specimens. Although there are wide species variations, the cartilaginous and osseous portions of the external acoustic meatus are lined by a thin epidermis formed by stratified squamous epithelium and a thin dermis that contains a relatively uniform allotment of sebaceous glands, fewer hair follicles, and greater ceruminous glands when comparing medial to lateral portions (Fig. 20-4). Sebaceous glands are composed of 6 to 10 club-shaped acini surrounded by thin fibrous tissue with ducts opening into associated hair follicles. Relative to ceruminous glands, they tend to be located in the more superficial dermis. Ceruminous glands are simple, coiled, tubular glands that resemble apocrine sweat glands. Their ducts open either into hair follicles or directly to the epidermal surface. Fig. 20-4 External acoustic meatus epithelium and adnexa, pig. Tympanic Membrane (Tympanum): The tympanic membrane, also known as the tympanum, is an extremely thin, three-layered, semitransparent membrane peripherally suspended from the tympanic ring by a fibrocartilaginous ring. The tympanic membrane is formed when the endoderm of the first pharyngeal pouch comes into close contact with the ectoderm of the first pharyngeal cleft or groove. Most of the tympanic membrane is held under tension so it can be deformed by and responsive to sound waves (Fig. 20-5). It covers the medial extremity of external acoustic meatus, demarcating the junction between the external ear and middle ear. Both surfaces of the tympanic membrane have an air interface. Fig. 20-5 Tympanic membrane. In most species, the tympanum is an oval-to-round structure, whereas in ruminants, it is shaped more like a broad triangle (Fig. 20-6). Embedded in the tympanum is the manubrium of the malleus. The placement of the manubrium in the tympanic membrane is highly variable between species. It is more centrally located in horses versus a more rostromedial location in ruminants and pigs. Fig. 20-6 Tympanic membrane. The tympanum is divided into two sections, the pars tensa and the pars flaccida. The majority of the tympanic membrane is made up of the pars tensa, which is a very thin, translucent, and taut membrane that bulges convexly into the tympanic cavity (Fig. 20-6, E). The pars tensa is made up of three layers: (1) outer layer of keratinizing squamous epithelium derived from ectoderm of the first pharyngeal groove; (2) middle layer of thin, variably vascularized fibrous connective tissue originating from the pharyngeal wall; and (3) inner layer of very low cuboidal to nonkeratinizing squamous epithelium, which is of pharyngeal pouch origin. The dorsal most portion of the tympanic membrane is the pars flaccida, which is roughly triangular, thicker, more vascular, and flaccid when compared to the pars tensa (see Fig. 20-6, E). Overlain by keratinized epithelium, the underlying stroma of the pars flaccida in dogs is made of loosely arranged collagen, rare mast cells, and few elastin fibers. This latter feature is in direct contrast to humans in which there are abundant elastin fibers. Visualized from the external acoustic meatus, this portion of the tympanic membrane may bulge into or away from the middle ear. The tympanic membrane is placed roughly at a 45-degree angle relative to the central axis of horizontal portion of the external acoustic meatus (Fig. 20-7, A). However, commonly, the actual placement of the tympanic membrane is more variable with the external, concave surface of the tympanic membrane angled more rostrally (Fig. 20-7, B and C). Cats have a similar orientation to their tympanum (Fig. 20-8). Interestingly, the surface area of the tympanic membrane from an approximately 550-kg horse is larger than the tympanic membrane of a Maltese dog but is approximately 15% smaller than the surface area of the tympanic membrane of a German shepherd dog. Additionally, the tympanum of the fetal goat is approximately 20% larger than that of large dog breeds. Fig. 20-7 External acoustic meatus, tympanic membrane, tympanic cavity. Compare unlabeled contralateral side with labeled side for more structural detail. Fig. 20-8 Tympanic bullae, cat. Tympanic Cavity: The tympanic cavity is an air-filled compartment surrounded by bone that is separated from the external ear by a thin tympanic membrane (tympanum) and is in direct communication with the pharynx via the auditory tube (also known as the eustachian or pharyngotympanic tube). Both the tympanic cavity and the auditory tube are derived from the endoderm of the first pharyngeal pouch. The epitympanic recess is the dorsal extremity of the tympanic cavity within which lies the head of the malleus and short curs of the incus. Ligaments stabilize and anchor the incudomallearis joint and the short crus of the incus within this recess (Fig. 20-9). Fig. 20-9 Malleus, incus, incudomallearis joint, epitympanic recess. In many species, there is a bulbous, ventral portion of the tympanic cavity called the tympanic bulla (see Fig. 20-3, B and C). Within the bulla of the dog and cat is a bony septum referred to as the septum bulla. In the cat, the septum bulla abuts the petrous portion of the temporal bone and separates the tympanic cavity into two compartments: the dorsolateral epitympanic cavity and the ventromedial tympanic cavity (see Fig. 20-8). This separation is incomplete, which allows communication between the two compartments through a narrow opening between the septum bulla and petrous portion of the temporal bone and a larger opening at its caudal edge. In the dog, this septum is a much smaller, incomplete bony ridge that only makes contact with the petrous portion of the temporal bone rostrally and often has tiny, elongate bony spicules with bulbous ends (see Fig. 20-7, B). The mucosal surfaces of the tympanic bullae of cats and dogs are lined by an epithelium that varies, depending on location. Dorsally, close to the auditory tube opening, the mucosa is composed mostly of ciliated columnar cells mixed with goblet cells and basal cells similar to the cells in the nasopharyngeal mucosae (Fig. 20-10 and see Fig. 20-20). Ventrally, the number of ciliated cells and goblet cells decreases and the number of cuboidal, less differentiated cells increases. The surfaces of the petrous portion of the temporal bone, auditory ossicles, and tympanic membrane are lined by cuboidal to noncornifying squamous epithelium. In ruminants, camelids, and pigs, the ventral portion of the tympanic cavity or bulla is made of more numerous bony compartments lined by noncornifying squamous epithelium (Fig. 20-11). In cattle and pigs, these compartments are air-filled with direct communication with the tympanic cavity. In camelids and small ruminants, the tympanic cavity does not appear to directly communicate with the bullae. Horses do not have readily identifiable tympanic bullae. Fig. 20-10 Tympanic cavity mucosa, cat. Fig. 20-11 Tympanic cavity and bulla. Auditory Ossicles: A chain of three bones or auditory ossicles forms the mechanical transduction system of hearing: the malleus, incus, and stapes (Figs. 20-12 and 20-13). The malleus and the incus, as well as the tensor tympani, are derived from the mesenchyme of the first branchial or pharyngeal arch. The stapes and the stapedius muscle originate from the mesenchyme of the second branchial or pharyngeal arch. Fig. 20-12 Schematic diagram of the middle and inner ear, dog. Fig. 20-13 Auditory ossicles and ossicular joints, cat. Malleus: The largest of the ossicles is the malleus. The manubrium of the malleus is embedded in the tympanic membrane (see Fig. 20-5). The most ventromedial convexity of the malleus is the “umbo” (see Figs. 20-6, F, and 20-12). The muscular process of the manubrium near the neck of the malleus is the attachment site of a thin tendinous portion of the tensor tympani muscle. Various ligaments stabilize the malleus in the epitympanic cavity by anchoring the long, thin rostral process, the neck, and the head of the malleus. The head of the malleus articulates with the articular surface of the body of the incus forming the incudomallearis joint (see Figs. 20-9, 20-12, and 20-13). In the horse and cow and in aged dogs and cats, the incudomallearis joint capsule is a narrow but thick ligament that makes disarticulation difficult and gives the external appearance of a falsely fused joint. In younger dogs and cats, the incudomallearis ligament is not nearly as tenacious and disarticulation is much less difficult. Incus: The incus is a bicuspid-shaped bone that lies caudal and dorsal to the malleus. It has two crura, one designated as the short crus, which is anchored in the epitympanic recess along with the body of the incus by a band of narrow connective tissue, and the other designated as the long crus, which transmits vibrations to the stapes. The lenticular process is at the end of the long crus (see Fig. 20-13), and in young animals, the lenticular process is a separate bone. In older animals, it fuses with the distal end of the long crus of the incus and articulates with the head of the stapes. Regardless of age, the incudostapedius joint capsular ligament is more translucent than the incudomallearis joint capsule and there is inherently more joint laxity. Stapes: The stapes, named for its close resemblance to stirrups of a saddle, is often considered the smallest bone in the body.* However, its size and shape is somewhat variable, depending on species (Fig. 20-14). Its base or footplate is convex and firmly seated in the oval or vestibular window of the petrous portion of the temporal bone and anchored by the annular ligament of the stapes. This arrangement forms a syndesmosis between the stapes base and cartilage of the oval window (Fig. 20-15). The stapedius muscle, fittingly referred to as the smallest muscle in the body, is attached to the muscular process of the shorter caudal crus close to the head of the stapes. Vibrations of the tympanic membrane are linearly transduced to stapes vibrations that lead to fluid waves of the perilymph of the internal ear. Fig. 20-14 Stapes, species variations. Fig. 20-15 Stapes in situ, horse. The middle ear has two muscles associated with the auditory ossicles that help modulate auditory transduction and a third muscle that controls patency of the auditory tube. The tensor tympani muscle originates rostrally and medially from the bony recess in the petrous portion of the temporal bone and makes its tendinous insertion onto the muscular process of the neck of the malleus (Fig. 20-16; see Fig. 20-13, A). It receives its innervation via a motor branch of the trigeminal nerve. Contraction of the tensor tympani muscle pulls the tympanic membrane medially and rostrally, placing greater tension on the auditory ossicle chain, which results in an increased resonant frequency of the auditory sound conduction system. Fig. 20-16 Auditory ossicular muscles. The stapedius muscle originates in the stapedius muscular fossa located dorsomedial to and obscured by the facial nerve as it courses through the facial groove of the temporal bone (Fig. 20-17). The stapedial branch of the facial nerve innervates this muscle as it converges into a thin tendon that inserts onto the muscular process of the short crus of the incus close to the head of the stapes. In the cat, the displacement variance of the stapes in its vestibular window is approximately 0.2 µm, whereas the maximal contraction of the stapedius muscle leads to dorsal and caudal stapedial bone displacement of between 40 and 60 µm. This displacement, which is perpendicular to the normal movement of the stapes, maximally attenuates sound transmission up to 30 decibels. Contraction of the stapedius muscle is an integral part of what is termed the acoustic reflex, defined as consensual, reflexive contraction of the muscle in response to stimuli (frequently sound) that leads to attenuated acoustic transmission. Fig. 20-17 Internal acoustic meatus, cat. Two cranial nerves provide motor branches to the muscles of the middle ear. Branches of the trigeminal nerve named for their respective muscles innervate the tensor tympani and tensor veli palatine muscles within the tympanic cavity. The facial nerve initially leaves the cranial cavity through the internal acoustic meatus (see Fig. 20-17), along with the vestibulocochlear cranial nerve, and then courses through the facial groove of the petrous portion of the temporal bone in close proximity to the oval window (Fig. 20-18). Several millimeters medial and lateral to the tendon of the stapedius muscle, the bony casing of the facial groove is incomplete, which allows direct communication between the epineurial connective tissue of the facial nerve and the tympanic cavity (see Fig. 20-18). This proximity and exposure of the facial nerve to the tympanic cavity explains why middle ear disease can manifest as facial nerve dysfunction. The facial nerve emerges from the middle ear through the stylomastoid foramen immediately caudal to the external acoustic meatus. Fig. 20-18 Stapedius muscle and its proximity to the facial nerve. In most mammalian species, the middle ear communicates with the pharynx through the auditory tube, which originates from the first pharyngeal pouch (Fig. 20-19). In the middle ear, the auditory tube opens into the most rostral and dorsal portion of the tympanic cavity called the epitympanic cavity. In the pharynx, the auditory tube originates from a narrow slit-like opening in the nasopharyngeal cavity and is lined by epithelium that is contiguous with the nasopharynx, namely ciliated, columnar pseudostratified epithelium mixed with goblet cells (Fig. 20-20). In many species, flanking the auditory tube are clusters of lymphocytes referred to as the tubal tonsil. Fig. 20-19 Auditory tube. Fig. 20-20 Histology of the auditory tube mucosa, cat. Unique to the horse and other Equidae, guttural pouches (see Chapters 9 and 17) are enlarged diverticula of the auditory tubes that extend further rostrally, medially, and ventrally when compared to auditory tubes of other mammalian species. Although the precise function of guttural pouches remains controversial, their proximity to internal carotid arteries and their ability to inflate during vigorous exercise makes the idea of an extracalvarial brain cooling apparatus a provocative hypothesis. The internal ear is essentially made up of several membranous compartments, collectively known as the membranous labyrinth, derived from ectoderm, that contain endolymph. The membranous labyrinthine compartments include the cochlea, sacculus, utricle, and each semicircular canal with associated ampullae (Fig. 20-21). Surrounding the membranous labyrinth is a protective bony shell, derived from mesoderm, known as the osseous labyrinth (i.e., petrous portion of the temporal bone). The membranous labyrinth is classically a rostrally coiled tube or cochlea with an intermediate compartment or vestibule and a caudal semicircular canal region. However, the shape of the petrous portion of the temporal bone is very different. The beginning of the cochlea is denoted by the prominent bulge in the petrous portion of the temporal bone known as the promontory. Caudally, portions of this bone give the appearance of the tubular membranous labyrinth that likely represent the semicircular canals. Within the membranous labyrinth are biologic mechanosensory hair cells (see later) responsible for hearing (the auditory compartment) and for assessing head position, acceleration, and balance (the vestibular compartment). Cochlea: The cochlea is the most complex portion of the membranous labyrinth comprising two closed-end tubular structures that are highly coiled (see Fig. 20-21). The central core of bony labyrinth around which the cochlea spirals nearly three times is the modiolus. Sound waves vibrate the tympanic membrane and are converted by coordinated movements of the malleus, incus, and stapes to fluid waves within the perilymph by vibrations of the vestibular or oval window. Fluid waves travel through the scala vestibuli toward the cupula, reaching the helicotrema, and then returning within the scala tympani toward the round (also known as cochlear) window. Positioned between the scala vestibuli and the scala tympani is the second closed compartment known as the cochlear duct or scala media. The cochlear duct is separated from the scala vestibuli by the vestibular membrane (also known as Reissner’s membrane), which transmits fluid waves of perilymph in the scala vestibuli into fluid waves of endolymph in the cochlear duct. Within the cochlear duct is a ribbonlike strip of extracellular matrix called the tectorial membrane. It is composed of several genetically distinct types of collagen (collagen types II, IX, and XI) and three distinct noncollagenous glycoproteins (α-tectorin, β-tectorin, and otogelin). The tectorial membrane rests on and is affixed to tips of hair cells that make up the mechanosensory portion of the organ of Corti that lies on the basilar membrane (Fig. 20-22). There are a series of three rows of outer hair cells and a single row of inner hair cells (see Fig. 20-21). Fluid waves in the cochlear duct result in movement of the tectorial membrane, which distorts inner and outer hair cells (neural-like cells), resulting in their depolarization and afferent transmission of action potentials via the cochlear branch of the vestibulocochlear cranial nerve to the vestibular nucleus. Fig. 20-22 Cochlear duct, cat. Vestibular System: The vestibular system is made of several endolymph-filled compartments located in the caudal third to half of the petrous portion of the temporal bone. It represents a major sensory system that (1) maintains balance in concert with general proprioception and visual systems, (2) coordinates body posture, and (3) helps maintain ocular position in relation to the position or motion of the head. Included in the vestibular system are the semicircular canals, utricle, saccule, vestibular ganglia, vestibular portion of cranial nerve VIII (vestibulocochlear nerve), vestibular nuclei, and vestibular lobules of the cerebellum. Portals of entry into the ear are listed in Box 20-1. Extension from the External Environment: Extension from the external environment is a common portal of entry into the external ear. It is a specialized invagination of the skin that terminates medially at the tympanic membrane of the middle ear. As the external acoustic meatus gradually narrows, its funnel shape is conducive in directing foreign materials, fomites, parasites, and/or infectious microorganisms into the external ear and toward the tympanic membrane of the middle ear. Additionally, its moist environment favors colonization of skin and/or mucosa by pathogenic microorganisms (Table 20-1). Although dermatitides can affect any part of the dermis, including the external ear, occasionally, involvement of the ear is an important feature used to arrive at a definitive diagnosis. TABLE 20-1 Predisposing Factors and Primary Causes of Otitis Externa Modified from Griffin CE, Kwochka KW, MacDonald JM: Otitis externa and media. In Current veterinary dermatology: The art and science of therapy, St. Louis, 1993, Mosby; and Scott DW, Miller WH, Griffin CE: Muller & Kirk’s small animal dermatology, ed 6, Philadelphia, 2001, WB Saunders. Hematogenous Spread: Hematogenous spread is a portal of entry into the external ear. Septicemias and/or specific types of viremias are thought to contribute to the development of external ear disease. Otitis externa and possibly deafness have been attributed to canine distemper virus, but it is unclear if the virus is a primary cause or one of several factors leading to otic disease. In an animal with septicemia, circulating bacteria have the potential to adhere to the endothelium of the capillary beds of the external ear, colonize the endothelium, and spread into adjacent tissues (see Chapter 4). Extension from the Middle Ear: Extension from the middle ear is another portal of entry into the external ear, especially in cavalier King Charles spaniels with primary secretory otitis media. In primary secretory otitis media, the external ear is typically unaffected unless the tympanic membrane is ruptured and mucoid debris is spread into the external acoustic meatus. However, it has been suggested that this breed may have underlying failure of mucosae of the middle ear predisposing these spaniels to auditory tube dysfunction and thus otitis media leading to rupture of the tympanic membrane. Extension through Perforation of the Tympanic Membrane: Extension from the external ear through a perforated tympanic membrane is a portal of entry into the middle ear (Fig. 20-23). In dogs with chronic otitis externa, secondary otitis media may occur in as many as 80% of affected dogs. At the time of clinical diagnosis, the tympanic membrane is most often intact, although some studies report perforations in over 40% of cases with otitis externa and concurrent otitis media. Based on the results of bacteriologic studies, it has been shown that a majority of dogs with concurrent otitis externa and otitis media have different bacteria isolated from each compartment. Thus it is unclear if the portal of entry in otitis media involves perforation of the tympanic membrane and spread of otitis externa into the middle ear with subsequent healing of the tympanic membrane or if otitis media results from bacteria ascending a poorly functioning auditory tube. Multiple studies implicate the latter mechanism (see later). Fig. 20-23 Ruptured tympanic membrane, dog. Ascension of the Auditory Tube: Ascension up the auditory tube is a portal of entry into the middle ear. It appears that dysfunction of the auditory tube is a necessary precursor for development of otitis media and likely leads to impaired clearance of middle ear effusions and prolonged periods of negative pressure within the tympanic cavity. Dysfunction may be related to impairment of the opening of the auditory tube into the pharynx during swallowing when pressure equalization takes place or it could be related to alterations of mucociliary clearance facilitated by epithelial cells lining the tube. Via either mechanism, it appears that microorganisms can use this portal to reach the middle ear. Extension Via Degeneration of the Temporohyoid Joint: Direct extension into the middle ear can occur from degeneration of the temporohyoid joint and release of microorganisms. See the discussion on temporohyoid osteoarthropathy in the section on Disorders of Horses. Extension Via Erosion through the Tympanic Bulla: Erosion through the tympanic bullae is a rare portal of entry into the middle ear. Neoplastic processes, such as oral squamous cell carcinomas, regional lymphosarcoma, or local compression by an abscess, can lead to bone remodeling, as well as bone lysis, with subsequent spread into the middle ear. Migration along Vascular or Neural Pathways: Branches of the caudal auricular artery and the facial nerve traverse within the middle ear and have the potential to serve as pathways for spread of microorganisms and neoplasms from the brain to the middle ear. This migratory process likely occurs via the extracellular matrix of arteries and nerves (see Chapters 3, 10, and 14) and via anterograde axonal transport in nerves (see Chapter 14). As an example, cranial nerve sheath tumors (see Chapter 14) in the brain have been reported to migrate along branches of the facial nerve and vestibulocochlear nerves and enter the ear via the internal acoustic meatus. Extension from the Middle Ear: Extension from the middle ear is a portal of entry into the internal ear, thus otitis interna or labyrinthitis is most commonly thought of as occurring by direct extension from an infection of the middle ear. The most likely portal is the round or cochlear window. Based on studies in cats, the permeability of the round window is increased for elements (sodium) and macromolecules (tritiated albumin) during mild cases of experimentally induced otitis media. Penetration through the oval or vestibular window is less likely because of the annular syndesmosis that is formed between the petrous portion of the temporal bone and the stapes (Fig. 20-24). Although otitis media may be diagnosed in isolation, otitis interna is rarely diagnosed without concurrent otitis media. Fig. 20-24 Chronic otitis media, cat. Hematogenous Spread: Entry through hematogenous spread occurs in the internal ear (see the previous discussion on the middle ear in the section on Portals of Entry for details). The ear’s responses to injury are listed in Box 20-2. The external ear is an extension of the integument, and it responds to inflammatory stimuli similarly. All of the hallmarks of inflammation occur in otitis externa. Initially, there is reddening and warmth of the affected auricle associated with otitis externa caused by vascular dilatation and hyperemia. Transudation of fluid out of leaky vessels leads to edema affecting both the auricle (see Fig. 17-13) and external acoustic meatus. Edema within the tissues results in swelling of the tissues and discomfort when the tissues are touched. As the inflammatory response progresses, the transudate becomes an exudate, infiltrating the dermis of the external ear. Epithelial and adnexal changes, described later, result in further expansion of the external ear dermis. Eventually, the lumen of the external acoustic meatus may become so stenotic that hearing function becomes impaired. As has already been described, the external acoustic meatus and auricle are lined by haired skin. Large, abundant, multiple, branching, and actively secreting sebaceous glands are most prominent in the deeper portions of the external acoustic meatus associated with hair follicles (see Fig. 20-5, A). Smaller, tubular, eccrine sweat glands, referred to as ceruminous glands, are located in the deeper layers of dermis. The epidermis, which is best studied in dogs, in response to inflammation, becomes hyperplastic and hyperkeratotic, although in some conditions it becomes ulcerated. Glandular changes include smaller, less abundant, less active sebaceous glands and more numerous, typically large, dilated ceruminous glands. Neutrophils, lymphocytes, and macrophages typically infiltrate the dermis, as well as the ectatic ceruminous glands (Fig. 20-25). Aggregates of lymphocytes form when the process is chronic. With increased chronicity, there is greater infiltration by fibroblasts and collagen, which can lead to more permanent stenotic changes. Finally, soft tissues, such as the dermis or auricular cartilage, may become ossified through metaplasia in the most chronically inflamed ears. Fig. 20-25 Otitis externa, external acoustic meatus, dog. Auricles of lightly pigmented cats chronically exposed to ultraviolet (UV) light are prone to developing squamous cell carcinoma (described later in the chapter; also see Chapter 17). UVB light leads to cellular transformation of the epithelial cells leading to a clonal population of neoplastic squamous epithelial cells. Myringitis: Inflammation of the tympanic membrane is called myringitis and is most commonly caused by bacterial infection of the external or middle ear (see Fig. 20-39, B). Macroscopically, the tympanic membrane may be congested, hemorrhagic, or thickened, resulting in opacity. Microscopically, the tympanic membrane has all of the characteristics of acute inflammation and if the inciting cause is unresolved results in chronic inflammation (see Chapter 3). Prolonged and severe myringitis may lead to perforation of the tympanic membrane and prevent its healing. Healing of the Tympanic Membrane: The tympanic membrane is a regionally well-vascularized membrane that has an air-interface along each surface. It can be perforated by traumatic injury, sudden exposures to high pressures, degradative enzymes and pressures from acute and chronic inflammation, chronic mite infestations, and neoplasms. Unique to the tympanic membrane, and likely related to its function, is its inherent ability to heal rapidly while maintaining its thin structure during healing through a process called epithelial migration. Unlike most other tissues, in which granulation tissue forms and bridges the defect followed by reepithelialization, the tympanic membrane closes the defect, first with migrating epithelial cells followed by a granulation tissue response that closes the mesenchymal portion of the tympanic membrane. Goblet Cell Metaplasia and Impaired Mucociliary Clearance: An important defense mechanism of the middle ear is the mucociliary apparatus of the auditory tube and contiguous tympanic cavity. Chronic otitis media causes a decrease in the number of ciliated cells (ciliary atrophy) in the mucosa of the auditory tube. Studies in cats determined that neutrophil lysate (likely consisting of degradative enzymes) or lipopolysaccharide did not significantly reduce the number of ciliated cells in the auditory tube, but auditory tube obstruction and likely increased pressure in the middle ear resulted in a dramatic decrease in the number of ciliated epithelial cells. In the same study, there was a marked increase in the total number of goblet cells lining the tympanic cavity and bulla. It was hypothesized that obstruction of the auditory tube elevated partial pressure of carbon dioxide (pCO2) in the mucus layer of the mucosa, triggering mucosal stem cells to differentiate toward goblet cells rather than ciliated cells. The viscoelasticity of the mucus produced by these goblet cells was greater than normal and resulted in a marked decrease of mucociliary clearance of the middle ear, probably further contributing to auditory tube obstruction, increased pressure in the middle era, and metaplasia of ciliated cells to goblet cells. Osteosclerosis of the Tympanic Bulla: In infections of the middle ear caused by microorganisms, mediators of acute and chronic inflammation, such as cytokines and degradative enzymes, may lead to excessive periosteal proliferation of new bone and osteosclerosis (thickening) of the wall of the tympanic bulla (Fig. 20-26; see Chapter 16). The bulla may become grossly distorted, and the lumen volume typically decreases. Inflammation also can contribute to bone lysis. Fig. 20-26 Chronic otitis media with osteosclerosis, guinea pig. Formation of Inflammatory Polyps: Inflammatory polyps are discussed later but are thought to represent both inflammatory and hyperplastic responses to injury induced by otitis media. Inflammation of the middle ear results in the formation of polypoid masses by an undetermined mechanism. These masses then result in clinical disease referable to where they exert their greatest effect. Polyps that involve the auditory tube and the nasopharynx can result in dysphagia and dyspnea, whereas those involving the tympanic membrane and external acoustic meatus lead to signs of otitis externa. Horner’s Syndrome/Pourfour Du Petit Syndrome: Postganglionic sympathetic fibers that innervate the eye course through the middle ear with branches of the internal carotid artery via the tympano-occipital fissure. Smooth muscle that lifts the upper eye, retractor muscles of the third eyelid, smooth muscle that tonically pulls the eyes rostrally, and dilator muscles of the pupil are all innervated by these fibers. Thus, in otitis media, cells and mediators of inflammation can act on postganglionic sympathetic fibers that innervate the eye as they traverse through the middle ear, leading to primary demyelination and axonal injury. Sensory Cell Degeneration/Death: Within the organ of Corti are the outer and inner sensory hair cells, as well as spiral ganglion neurons. The sensory hair cells are the most vulnerable to injury. Damage to the sensory cells or ganglion cells results in impaired function that is often permanent. Whether the cause is labyrinthitis, noise-induced hearing loss (85 dB sound pressure level or higher), chemical ototoxins, or the poorly understood disorder called presbycusis (i.e., age-related hearing loss), sensory hair cells are targeted and undergo degeneration or death leading to hearing impairment or hearing loss. Bursts of intense noise are thought to result in disarrangement or breakage to the stereocilia, whereas continuous exposure to damaging noise leads to death of the hair cells. Auditory Ossicular Chain Damage: Chronic otitis media can potentially lead to conductive hearing loss by damaging the auditory ossicles. Chronic exposure to infection, bacterial or fungal toxins, and degradative enzymes from inflammation can result in bony lysis. Excessively lytic bones may develop pathologic fractures. Additionally, chronic inflammation can result in fibrosis, which may restrict the movement of the ossicles. Finally, chronic inflammation can damage the articulations of the ossicles, thus impairing transmission of vibrations. Defense mechanisms of the ear are listed in Box 20-3. Epithelial Migration: Migration of cornified epithelial cells to the periphery of the tympanic membrane and onto the external acoustic meatus continuously replaces the effete epidermal layer of the tympanic membrane. Epithelial migration is thus an important means of maintaining tympanic membrane thickness, clearance of debris and likely microorganisms and parasites from the tympanic membrane and external acoustic meatus, and retaining vibratory sensitivity. Alterations in this epithelial migratory pattern can lead to or may be the cause of disease of the external or middle ear. As discussed previously, epithelial migration is also the method by which perforations of the tympanic membrane are repaired. Adnexa and Cerumen: Cerumen is an oily emulsion that coats and protects the integument of the external acoustic meatus. Its naturally hydrophobic properties make it an important barrier to the entry of excessive moisture into the epidermal cells or underlying dermis. In normal ears of dogs, cerumen is made of sloughed superficial squamous cells mixed with ceruminous and sebaceous gland secretions. There is a high lipid content in cerumen made up of neutral lipids. In otitic ears, the cerumen changes because ceruminous glands are typically more numerous and active during periods of inflammation and thus contribute more to the content of cerumen (see Fig. 20-25). One consequence is a decrease in the lipid content, a decrease in hydrophobicity, and impairment of a natural barrier. Commensal Organisms: A mixture of bacteria and yeast normally populates the external acoustic meatus. Various species of “potentially pathogenic” microorganisms have been cultured from external ears of dogs with no evidence of disease. They include Bacillus spp., Corynebacterium spp., Escherichia coli, Micrococcus spp., Staphylococcus spp., Streptococcus spp., and yeast organisms, most commonly Malassezia spp. Rarely, Pseudomonas spp. and Proteus spp. have been isolated. Based on a single study of horses, Corynebacterium spp. and Staphylococcus intermedius were isolated from normal ears. Presumably, these organisms live symbiotically and defense mechanisms limit or prevent proliferation of a monoculture. However, the spectrum of microorganisms cultured from diseased external ears closely mirrors this list. Osseous External Acoustic Meatus: The length and diameter of the osseous portion of the external acoustic meatus are highly variable among species and provide a unique structural defense mechanism. Animals with longer and narrower osseous portions have tympanic cavities and tympanic membranes that are better protected than those with shorter osseous portions. Mucociliary Apparatus: Various portions of the middle ear are lined by epithelium similar to that found in the nasopharynx. A combination of goblet cells and ciliated columnar epithelial cells extend through the auditory tube into the tympanic cavity. See Chapters 4 and 9 for discussion of the mucociliary apparatus. Surfactant: Surfactant in the lung has long been recognized for its central role in reducing surface tension in alveoli, allowing them to expand and collapse normally during inspiration and expiration, respectively (see Chapter 9). However, recently, it has been shown that surfactant has other roles, especially in the function of the auditory tube. Surfactant is a complex mixture made up of 90% lipid and phospholipid and 10% surfactant proteins, designated surfactant protein-A (SP-A), SP-B, SP-C, and SP-D. Cuboidal epithelial cells lining the auditory tube are the source of surfactant and contain apical secretory granules analogous to what are seen in type II pneumocytes of the lung. One major difference between pulmonary surfactant and auditory tube surfactant is the ratio of phosphatidylcholine to sphingomyelin. Pulmonary surfactant has a ratio of 67 to 1, whereas auditory tube surfactant has a ratio of 2 to 1. This dramatic difference in auditory tube surfactant phospholipid content is reflected in a reduced ability to modify surface tension. Surfactant proteins are collectins that have two domains: (1) a lectin moiety that binds to the surface of foreign substances and organisms and (2) a collagenous domain that functions as a ligand for phagocytosis and complement activation (see Chapter 3). Finally, surfactant is believed to play a protective role against free radicals by early termination of free radical propagation, and protecting against oxidative injury. A lipid-binding pocket in surfactant proteins may prevent free radicals from propagating.
The Ear and Eye*
Ear
Structure And Function
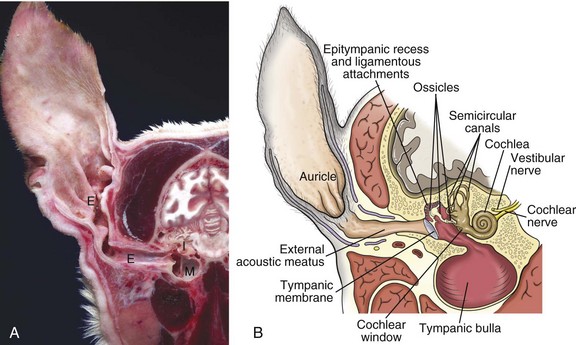
A, Cross-section, head through the right ear, rostral surface, dog. External (E), middle (M), and inner (I) ear are illustrated. The tympanic membrane has been removed in this section. The external ear consists of the auricle and external acoustic meatus; the middle ear consists of the ossicles, tympanic cavity, and bulla; and the inner ear consists of the cochlea and semicircular canals. B, Schematic diagram depicting a cross-section through the external, middle, and inner ear of a dog. (A courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
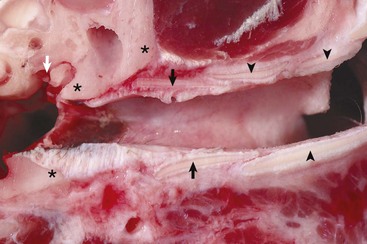
Annular (black arrows) and auricular cartilage (black arrowheads) form the structure of the cartilaginous portion of the external acoustic meatus. Dense, white, fibrous connective tissue attaches the annular cartilage to the osseous rim of the external acoustic meatus (asterisks). The incudostapedius joint is visible in this image (white arrow). The tympanic membrane is removed in this section. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
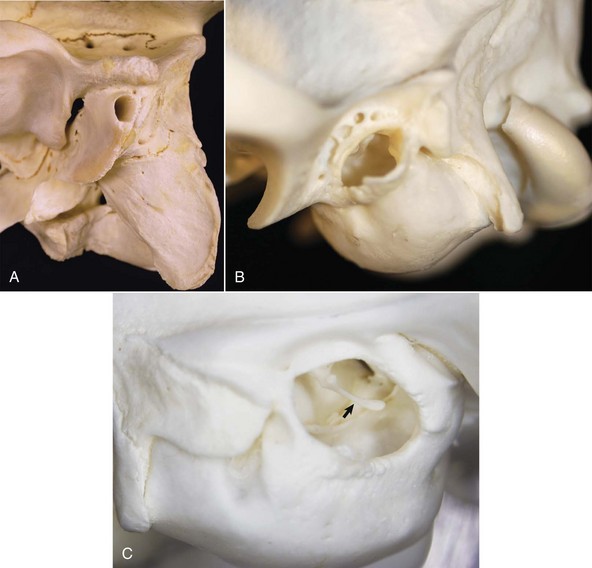
A, Ox. The bony external acoustic meatus in most livestock is much longer and narrower when compared to dogs and cats. Visualization of the middle ear is obscured by the elongate osseous external acoustic meatus. B, Dog. The osseous portion of the external acoustic meatus is a thin rim of bone allowing easy visualization of the middle ear. C, Cat. The osseous portion of the external acoustic meatus is very thin and the opening is very large, allowing easy visualization of the middle ear and malleus (arrow). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
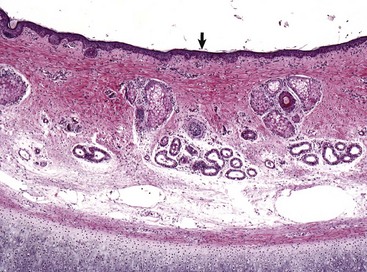
Stratified squamous epithelium (arrow) lines the external acoustic meatus. Adnexa present are mixtures of sebaceous glands, most often flanking hair follicles, and deeper eccrine glands referred to as ceruminous glands. Auricular cartilage is present along the lower edge of the image. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Middle Ear
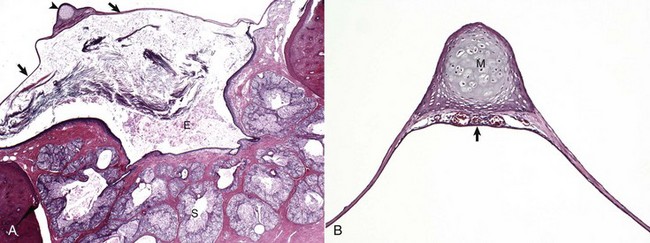
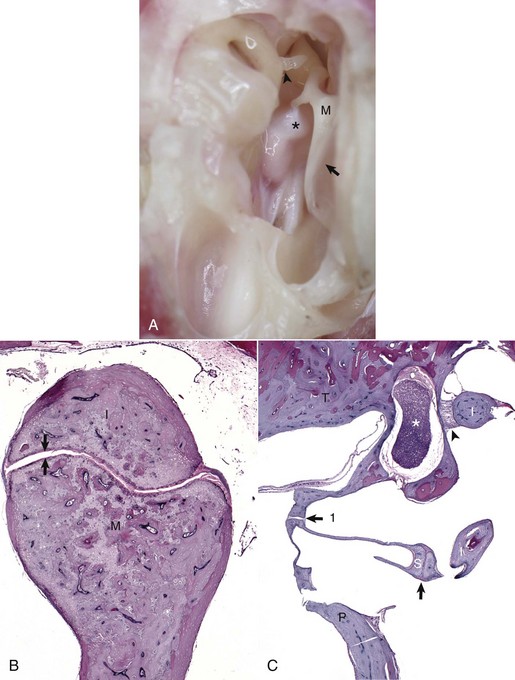
A, Cat. Cross-section through the tympanic membrane (arrows), manubrium of the malleus (arrowhead), and external acoustic meatus (E). An intact tympanic membrane at the level of the pars tensa is very thin. It is lined by a single layer of cornified squamous epithelial cells externally, and low cuboidal to noncornified squamous epithelium line the inner surface. The abundant keratin within the external acoustic meatus is not uncommon. The prominent, multiple, branching sebaceous glands (S) are common in the deepest portions of the external acoustic meatus. H&E stain. B, Higher magnification of the tympanic membrane and malleus, pig. The manubrium (M) of the malleus is embedded in the tympanic membrane. Numerous blood vessels are present beneath the manubrium (arrow) and are located in the middle layer of the tympanic membrane beneath the external concave surface, which corresponds with the region of germinative epithelium. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
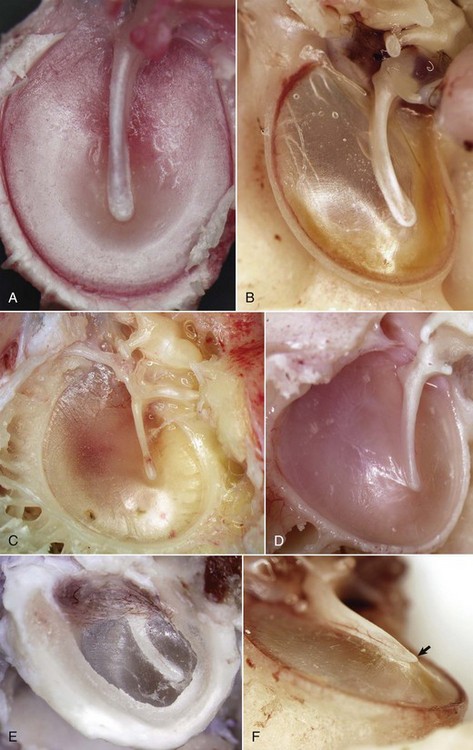
A, Horse. In the horse, the tympanic membrane (tympanum) is more round than other species. The manubrium of the malleus forms a very shallow arc and is centrally located in the tympanum. B, Dog. The tympanum is oval to comma-shaped in dogs, and the manubrium of the malleus is C-shaped. C, Pig. The shape of the tympanic membrane is similar to that of the dog, but the manubrium of the malleus is shorter and straighter. D, Goat. Ruminants tend to have a more triangular-shaped tympanic membrane. In both pigs and ruminants, the manubrium of the malleus is more rostral and medial than in other species. E, Dog. Lateral view of tympanic membrane. The transparent portion of the tympanic membrane held under tension and associated with the manubrium of the malleus is the pars tensa. The highlights depict the radial striations of the normal pars tensa. Dorsally, the tympanic membrane is thicker, highly vascular and is under much less tension, and is designated as the pars flaccida. F, Dog. The tympanic membrane is convex on its medial surface in the tympanic cavity. The ventromedial extremity of the manubrium is called the umbo (arrow). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
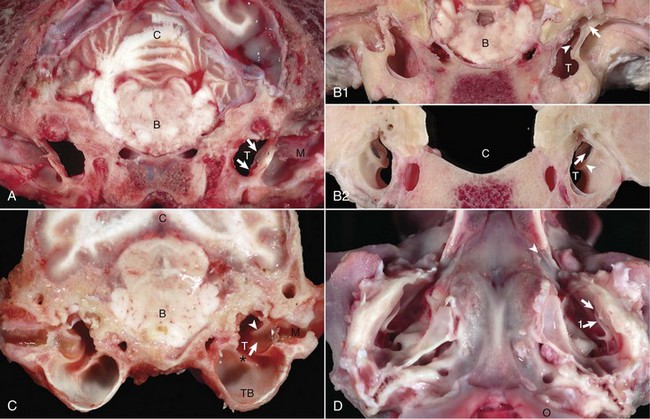
A, Transverse section, rostral surface, dog. The tympanic membrane (arrows) extends medially towards the tympanic cavity (T) at an approximate 45-degree angle from dorsal to ventral, in relation to the central axis of the horizontal part of the external acoustic meatus (M). Portions of the rostral edge of the tympanic ring have been inadvertently removed during sample preparation. Brainstem (B); cerebellum (C). B, Transverse section, rostral (1) and caudal (2) surfaces, goat. The tympanic cavity (T) has been opened bilaterally. In the cranial view (1), the tympanic membrane is not visualized because it is hidden by the tympanic ring (arrows), which surround the membrane. The manubrium of the malleus (arrowheads) is minimally visible. However, from the caudal view (2), the tympanic membrane (arrows) is clearly visible and is positioned so that the concave or external surface is tilted rostrally. Brainstem (B); calvarium where brainstem would be positioned (C); tympanic ring (arrowheads). C, Transverse section, caudal surface, dog. The external or concave surface of the tympanic membrane (arrow) is angled almost fully rostral rather than lateral. Note that the septum bullae (asterisk) are short and incomplete in the dog when compared with a cat (see Fig. 20-10). Brainstem (B); Cerebellum (C); Tympanic cavity (T); Manubrium of the malleus (arrowheads); External acoustic meatus (M), Tympanic bullae (TB). D, Ventral-dorsal view, opened bullae. Bilaterally, the concave surface of the tympanic membranes (arrow) are tilted rostrally. Occipital condyles (O) appear at the bottom of the image. Extending rostrally and medially from the tympanic cavity are the auditory tubes (arrowhead), which provide direct communication between the tympanic cavity and nasopharynx. Manubrium of the malleus (arrow 1). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
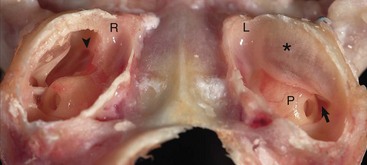
Caudoventral section, with both tympanic bullae opened ventrally. The septum bulla (asterisk) is intact in the left bulla (L) and opened ventrally in the right bulla (R). From rostral to caudal, the septum bulla dorsally abuts the petrous portion of the temporal bone. At its caudal extreme is an opening that allows communication between the two cavities (arrow). The auditory tube opening into the tympanic cavity is observed in the dorsal, rostral extremity of the right epitympanic cavity (arrowhead). The large bulge rostral to the round window corresponds to the start of the cochlea and is called the promontory (P). In both specimens the tympanic membrane is tilted rostrally. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
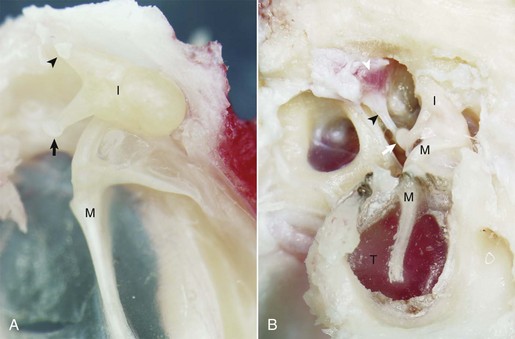
A, Cat. Medial view. Seated in the epitympanic recess is the rounded head of the malleus (M) and the incus (I); together they are articulated to form the incudomallearis joint. The smaller crus of the incus (arrowhead) and the head of the malleus are anchored to the epitympanic recess by ligaments (see Fig. 20-1). At the end of the longer crus of the incus is the lenticular process (arrow). B, Giraffe. Lateral view, right ear. The tympanic membrane is intact (T). The small crus of the incus (I) along with the head of the malleus (M) are firmly anchored in the epitympanic recess by ligamentous attachments. The lenticular process of the long crus articulates with the head of the stapes seated in the oval window to form the incudostapedius joint (white arrow). The facial nerve has been removed in order to expose the stapedius muscle (white arrowhead), which is firmly attached to the stapes via its tendon (black arrowhead). (Courtesy Dr. B.L. Njaa, The Center for Veterinary Health Sciences, Oklahoma State University.)
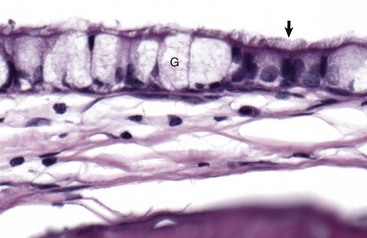
The mucosa of the tympanic cavity is not uniform. In cats and dogs, the mucosal epithelium in the more dorsal portions of the bullae morphologically mirrors mucosae of the nasopharynx. Included are ciliated columnar epithelial cells (arrow) and goblet cells (G) mixed with fewer nonciliated columnar epithelial cells. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
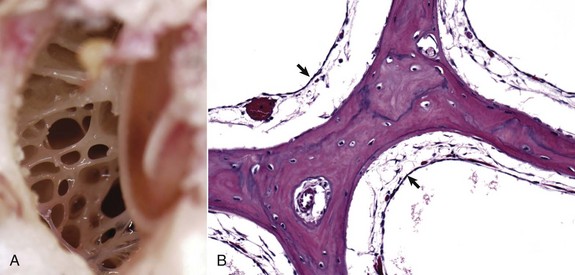
A, Dorsal view into the middle ear, ox. Ruminants and pigs have small tympanic cavities but much larger tympanic bullae. The bullae are made of multiple, arborizing air-filled channels with numerous bony septa as shown here. B, Histologic section of tympanic bulla, pig. Bony septa are lined by low cuboidal to nonkeratinizing squamous epithelial cells (arrows). H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
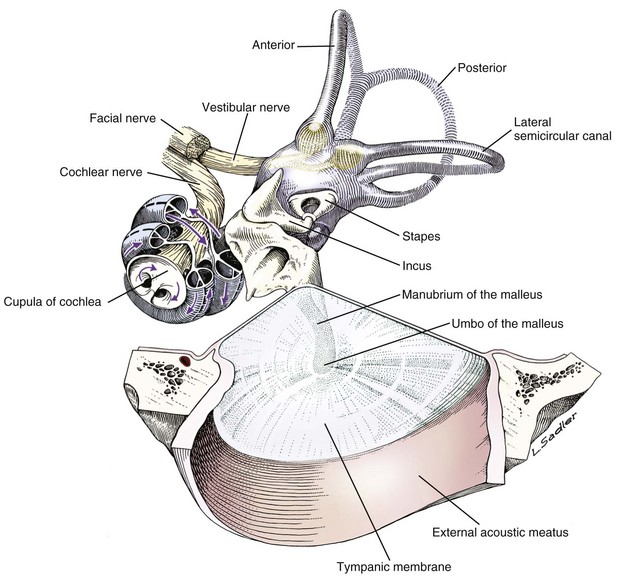
Tympanic membrane, auditory ossicles, and membranous labyrinth. The bony labyrinth has been removed to demonstrate the orientation of the cochlea and semicircular canals relative to the auditory ossicles and tympanic membrane. The facial nerve and vestibulocochlear nerve enter the ear together through the internal acoustic meatus.
A, Tympanic cavity, tympanic membrane, auditory ossicles, petrous portion of the temporal bone, auditory muscles, ventral view. The manubrium of the malleus is embedded in the tympanic membrane. The head of the malleus and incus are anchored in the epitympanic recess and form the incudomallearis joint. The long crus of the incus is shown articulating with the stapes to form the incudostapedius joint (arrowhead). Attached to the muscular process of the malleus (M) is the tensor tympani muscle (asterisk). Tympanic membrane (arrow). B, Histologic section of the incudomallearis joint (between the arrows), normal. The articulation of the malleus (M) and incus (I) is shown in the epitympanic recess. Similar to the petrous portion of the temporal bone, these ossicular bones lack a medulla. C, Histologic section of the incudostapedius joint. Positioned in the oval window is the stapes (S), held in place by a syndesmosis (arrow 1). The more ventral edge of the stapes has artifactually fractured. Articulating with the head of the stapes is the lenticular process of the long crus of the incus (I) to form the incudostapedius joint (arrow). The lenticular process of the incus is to the right of the incudostapedius joint. A portion of the short crus is positioned in the epitympanic recess and anchored by a ligamentous attachment (arrowhead). The facial nerve is present coursing through the facial groove (asterisk). Note the lack of complete bony encasement allowing communication with the tympanic cavity. Promontory (P), petrous portion of the temporal bone (T). H&E stain. (A and B courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
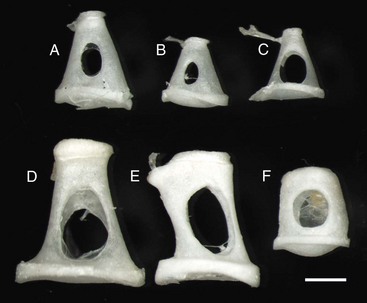
Stapes are highly variable in size and shape, depending on the species. The first two stapes, beginning from the top row left are from different sized dogs. The larger stapes is from a 20 kg mixed breed dog (A). The smaller stapes is from a Maltese dog (B). The third stapes of the upper row is from a cat (C). The lower row depicts stapes from a horse (D), cow (E), and slaughter-age pig (F). In all cases, the lower plate of the stapes is convex. Also, in each case, the tendinous attachment of the stapedius muscle is affixed to the shorter crus or limb of the stapes. Scale bar = 1 mm. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
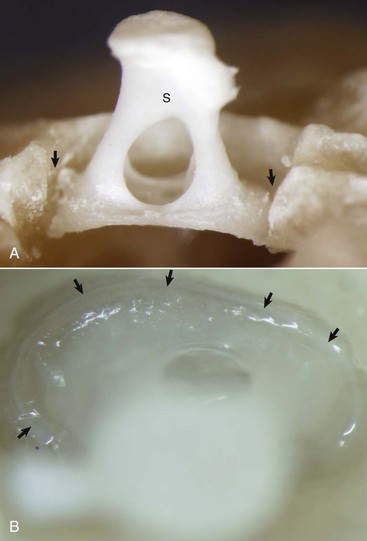
A, Partially opened oval window. The stapes (S) is seated in the oval window connected to the petrous portion of the temporal bone by the annular ligament (arrows). B, Ventrolateral view of the stapes, in situ. A thin rim of annular cartilage is visible denoting the syndesmosis formed between the stapes and cartilage of the oval window of the petrous portion of the temporal bone (arrows). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Middle Ear Muscles And Nerves
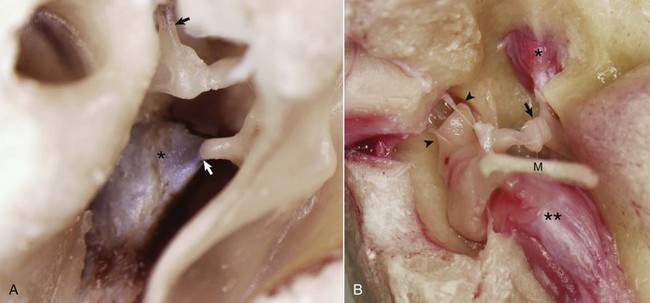
A, Middle ear, caudal view, ox. The external acoustic meatus is to the right on this image with the caudal edge of the tympanic membrane removed. The tensor tympani muscle (asterisk) is attached via its tendon to the muscular process of the malleus (arrow). The tendon of the stapedius muscle (arrowhead) is attached to the stapes bone near the head of the stapes. B, Middle ear, ventral view, horse. The bony external acoustic meatus, tympanic membrane, and associated connective tissue have been removed. The articulated incus and malleus form the incudomalleolar joint and are anchored in the epitympanic recess (arrowheads). The incudostapedius joint is opened (arrow). The facial nerve and some of the surrounding bone have been removed to expose the stapedius muscle (asterisk) attached to the stapes seated in the oval window. The muscular process of the malleus is obscured by the position of the manubrium (M) of the malleus, which is attached to the tensor tympani muscle (two asterisks). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
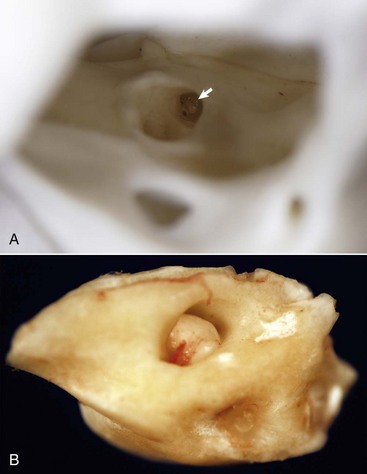
A, Right internal acoustic meatus. Viewed through the open left external acoustic meatus, the right internal acoustic meatus (arrow) represents the bony opening into the petrous portion of the temporal bone, through which the vestibulocochlear nerve and facial nerve exit the cranial cavity. B, Petrous portion of the temporal bone, cat. The yellow hue is very typical of this bone in all species. The large central opening is the internal acoustic meatus within which lie the vestibular and cochlear nerves. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
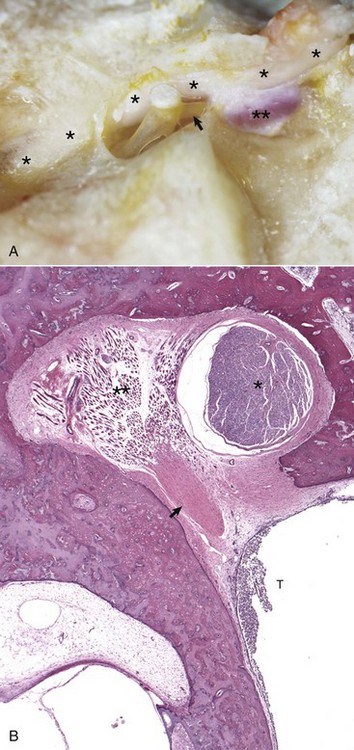
A, Horse. The facial nerve (asterisks) courses through the facial groove in close proximity to the stapes and partially obscures the stapedius muscle (two asterisks). The tendon of the stapedius muscle (arrow) is shown attached to the short arm of the stapes, near the head of the stapes. B, Dog. Histology of facial nerve and stapedius muscle. The facial nerve (asterisk) is present within the facial groove partially obscuring the stapedius muscle (two asterisks) anchored in its stapedius muscular fossa. The tendon of the stapedius muscle (arrow) is observed in oblique, transverse section, but the stapes is not in this plane of section. Within the tympanic cavity (T) are moderate numbers of neutrophils, indicative of suppurative otitis media. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Auditory Tube (Eustachian Or Pharyngotympanic Tube)
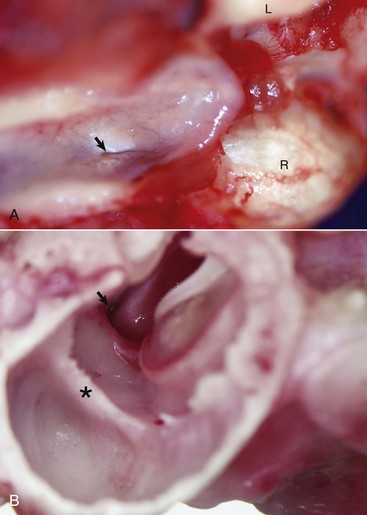
A, Nasopharynx, cat. A thin slit-like opening, normally maintained in a closed position, represents the opening of the right auditory tube into the nasopharynx (arrow). L, Left occipital condyle; R, right occipital condyle. B, Middle ear, tympanic bulla, right ear, caudal, oblique view, dog. The auditory tube (arrow) is located dorsal, medial, and rostral to the tympanic ring of the tympanic membrane just to the left of the tympanic membrane and manubrium of the malleus. The septum bulla (asterisk) is short and incomplete when compared to the cat. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
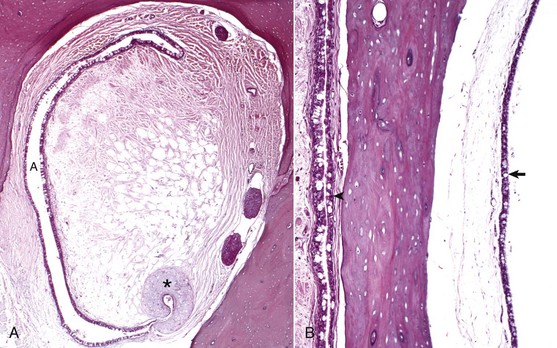
A, Rostral opening of the auditory tube. The auditory tube (A) in cross-section is typically C-shaped. Comma-shaped cartilage (asterisk) provides structural support to portions of the auditory tube. The mucosal lining is composed of ciliated, pseudostratified, columnar epithelium mixed with goblet cells and non-ciliated epithelial cells. H&E stain. B, Cross-section through tympanic bulla and auditory tube. The mucosae of the tympanic bulla (arrow) and the lining of the auditory tube (arrowhead) are composed of pseudostratified, ciliated columnar epithelial cells mixed with goblet cells and basal cells. This mucosa is virtually identical to the nasopharyngeal mucosa. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Internal Ear
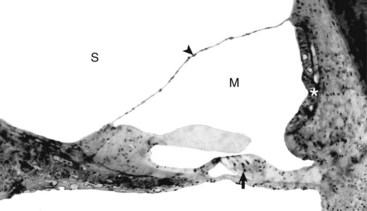
The tectorial membrane is positioned over the inner and outer hair cells of the organ of Corti (arrow). The intact vestibular membrane (arrowhead) becomes distorted when auditory ossicular movements create fluid waves in the perilymph of the scala vestibuli (S). The stria vascularis (asterisk) is the source of endolymph production and maintenance. See Fig. 20-21 for a diagram of the structure. M, Scala media. Thick tissue section, toluidine blue stain. (Courtesy Dr. D.A. Ryugo; from Ryugo DA, Cahill HB, Rose LS, et al: Hear Res 181:73-84, 2003.)
Portals Of Entry
External Ear
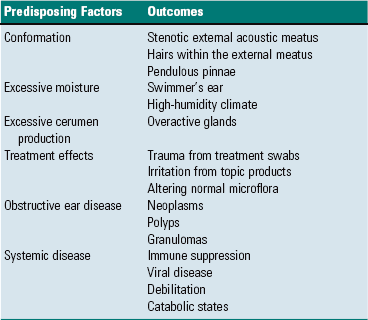
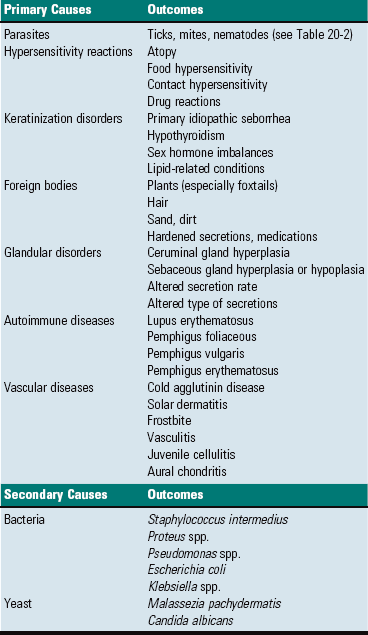
Middle Ear
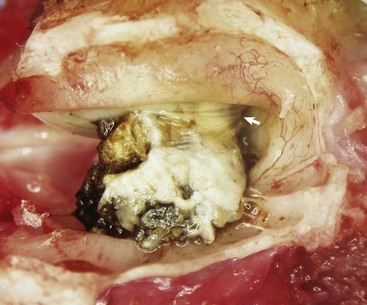
A large aggregate of cerumen bulges through a tear in the caudal portion of the pars tensa between the manubrium of the malleus and the caudal bony tympanic ring into the tympanic cavity. The wrinkled flap of the torn pars tensa can be seen (arrow). There was no evidence of otitis media and likely the tear was acute. A ceruminous gland adenoma was identified in the horizontal portion of the external acoustic meatus unassociated with the tympanic membrane causing complete obstruction. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Internal Ear
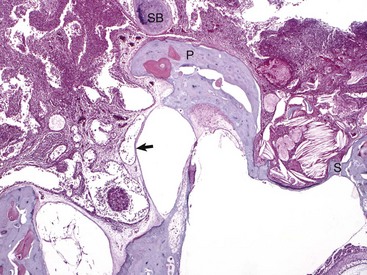
Histologic section of middle ear, petrous portion of the temporal bone through the round and oval window with the stapes in situ. The syndesmosis formed between the bone of the oval or vestibular window of the petrous portion of the temporal bone seems to prevent otitis media from spreading into the inner ear. Conversely, the membranous covering of the round or cochlear window is infiltrated by inflammatory cells. During episodes of otitis media, the permeability of this membrane is increased. Otitis interna was diagnosed in this cat (not depicted in this image). At the top of the image is the edge of the septum bulla abutting the petrous portion of the temporal bone, anatomic indication that this is from a cat. Edge of stapes (S); round window membrane (arrow); promontory (P); septum bulla (SB). H&E stain. (Courtesy Dr. B. L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Responses To Injury
External Ear
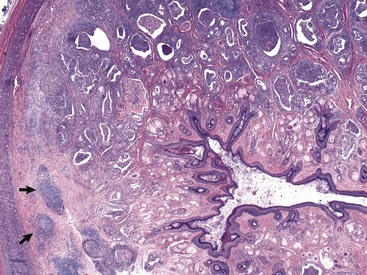
In this histologic section, the dermis contains increased numbers of ceruminous glands that are dilated and filled with inflammatory cells, typically neutrophils, macrophages, lymphocytes, and plasma cells. These inflammatory cells are also present in the periadnexal dermis. Lymphoid aggregates form nodules (arrows) in the deep dermis near the cartilaginous rings of the external acoustic meatus. The overlying epidermis is mildly to moderately thickened (acanthosis). The luminal diameter has been markedly reduced, which can further exacerbate the problem of otitis externa. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Middle Ear
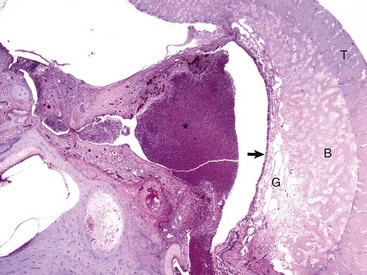
The tympanic cavity is partially filled with a suppurative exudate (asterisk, center of the image). The inner surface of the tympanic bulla is lined by a thickened hyperplastic mucosa (arrow). Below the mucosa is an area of granulation tissue (G) that overlies the formation newly remodeled bone (B), a reparative or healing response to injury. Tinctorially, the tympanic bulla (T) is the darker blue area along the right margin of the image. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Internal Ear
Defense Mechanisms
External Ear
Middle Ear
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Ear and Eye