CHAPTER 14 The Central Nervous System
CEREBROSPINAL FLUID
Cerebrospinal fluid (CSF) evaluation is a mainstay in the diagnosis of central nervous system (CNS) disease because it is relatively simple to collect and has the potential to provide valuable information. Lesions of the CNS do not consistently cause CSF abnormalities related to the location and extent of the lesion. Although CSF evaluation infrequently provides a definitive diagnosis, it may be of benefit in documenting normal or abnormal features and, in combination with other tests, determining a diagnosis or differential diagnoses (Bohn et al., 2006; Bush et al., 2002; Chrisman, 1992; Cook and DeNicola, 1988; Fenner, 2000; Rand, 1995). CSF collection is recommended as a part of virtually any diagnostic investigation of CNS disease of unknown cause when contraindications to its collection are not present.
Collection of Cerebrospinal Fluid
Contraindications to CSF Collection
Cerebrospinal fluid collection is not indicated in cases in which a cause is obvious, such as known trauma or intoxication (Parent and Rand, 1994). Because anesthesia is required for collection of CSF in small animals, CSF collection is contraindicated in cases in which anesthesia is contraindicated (Carmichael, 1998; Cook and DeNicola, 1988).
Cerebrospinal fluid collection is contraindicated in cases with increased intracranial pressure. Increased intracranial pressure should be suspected with acute head trauma, active or decompensated hydrocephalus, anisocoria, papilledema, or cerebral edema. Expansile mass lesions and unstable CNS or systemic conditions may result in increased intracranial pressure or decreased pressure in the spinal compartment relative to the intracranial compartment. In these situations herniation of the brain may result in severe compromise of brain function, tetraplegia, stupor/coma, and/or death (Parent and Rand, 1994). Physical and neurologic examination, history, presentation, and results of imaging studies are of benefit in determining if these conditions are likely before the decision to collect CSF.
Even in cases with potential for brain herniation, the risks associated with CSF collection may be acceptable if the cause of patient deterioration is not apparent. Risk of herniation may be reduced by administration of dexamethasone (0.25 mg/kg IV) just before induction of anesthesia and by hyperventilation of the patient with oxygen during the procedure (Fenner, 2000). Except in cases in which dexamethasone is administered prophylactically because of suspected increased intracranial pressure, CSF collection should predate corticosteroid administration because of potential alteration of CSF composition (Rand, 1995).
Complications of CSF Collection
As with any medical procedure, the risks and benefits of CSF collection should be considered for individual cases. The potential exists for iatrogenic trauma to the spinal cord and/or brainstem from the collection needle, but is minimized by attention to anatomic landmarks and careful collection procedures (Carmichael, 1998; Parent and Rand, 1994). Risk of introduction of infectious agents into the CNS is minimized by adherence to the basic principles of aseptic technique and correct preparation of the site of collection (Cook and DeNicola, 1988).
Slight to moderate blood contamination is a common complication of collection associated with penetration of the dorsal vertebral sinuses or small vessels within the meninges; this may complicate interpretation of the fluid analyses and cytology, but has not been found to be harmful to the patient (Carmichael, 1998; Fenner, 2000).
Ketamine should not be used to anesthetize cats for CSF collection because it increases intracranial pressure and may induce seizures; gas anesthesia should be used (Parent and Rand, 1994).
Equipment for CSF Collection
Clippers, scrub, and alcohol to surgically prepare the site of collection are needed. Sterile gloves should be worn during the procedure. A sterile disposable or resterilizable spinal needle with stylet is used. A 20- to 22-gauge, 1.5-inch needle with a polypropylene hub is recommended for most cases, although smaller needles may be needed in very small dogs and cats and longer needles may be needed in large dogs (Carmichael, 1998; Cook and DeNicola, 1988; Parent and Rand, 1994; Rand, 1995). Several needles should be available since replacement may be needed if the needle is inserted off the midline and enters a venous sinus (Cook and DeNicola, 1988).
Sterile plain tubes for collection of CSF are recommended. Some authors indicate that EDTA is not used because clotting is rare and EDTA may falsely elevate the protein concentration of CSF (Parent and Rand, 1994). However, others recommend addition of samples to EDTA if blood contamination is present or if elevated nucleated cell count, bacteria, elevated protein concentration, and/or the presence of fibrinogen are suspected because these may lead to clotting (Carmichael, 1998). If glucose determination is desired, it is recommended to collect CSF into fluoride/oxalate; this may not be necessary if CSF contains few erythrocytes and is analyzed rapidly.
Collection Volume
Carmichael (1998) indicates that approximately 1 mL of CSF per 5 kg of body weight can be collected safely. It may be dangerous to remove more than 1 mL of CSF per 30 seconds, more than 4 to 5 mL of CSF from the dog, more than 0.5 to 1 mL of CSF from the adult cat or more than 10 to 20 drops of CSF from the kitten. Rand (1990) indicates that 1.0 to 1.5 mL of CSF can usually be collected from the cat. The cat may be susceptible to meningeal hemorrhage if too much fluid is withdrawn.
Cerebellomedullary Cistern Collection
Preparation of the site should include clipping of the hair from the head and neck, from the anterior margin of the pinna to the level of the third cervical vertebra and laterally to the level of the lateral margins of the pinnae. This area should be scrubbed for a sterile procedure (Cellio, 2001).
The animal is positioned in lateral recumbency with the head and vertebral column positioned at an angle of approximately 90 degrees. Excessive flexion of the neck may result in elevation of intracranial pressure and increase the potential for brain herniation (Fenner, 2000) or may result in occlusion of the endotracheal tube (Carmichael, 1998). The nose should be held or propped so that its long axis is parallel to the table and it should not be allowed to rotate in either direction. The point of insertion is located on the midline approximately half way between the external occipital protuberance and the craniodorsal tip of the dorsal spine of C2 (axis) and just rostral to the anterior margins of the wings of C1 (atlas). The needle is inserted at the intersection of a line connecting the anterior borders of the wings of the atlas and a line drawn from the occipital crest to the dorsal border of the axis along the midline. Puncture of the skin first with an 18-gauge needle or a scalpel blade is helpful in overcoming skin resistance in thick-skinned animals. Alternatively, the skin can be pinched and lifted so that the needle can be safely pushed through the skin with a twisting motion.
If opening pressure readings are taken, CSF fluid sample is taken by directing the flow of CSF through the manometer by way of a three-way stopcock. If pressure readings are not obtained, CSF may be collected directly from the spinal needle hub by dripping into a test tube or gentle aspiration of drops as they collect at the hub using a syringe. Attachment of a syringe to the needle with aspiration of CSF is not recommended because suction may result in contamination with blood or meningeal cells or obstruction of CSF flow by aspirated meningeal trabeculae. Careful aspiration is acknowledged to be necessary for collections in some cases. Passage of the needle through the spinal cord to underlying bone should be avoided at the cerebellomedullary cistern because it may cause damage to the cord and/or cause blood contamination of the CSF sample. On completion of collection of CSF, the needle is smoothly withdrawn. Replacement of the stylet is not necessary.
Lumbar Cistern Collection
Both cerebellomedullary and lumbar cistern specimens may be collected. Collection of a cerebellomedullary specimen is recommended prior to thoracolumbar myelography to ensure that a diagnostic CSF sample will be obtained because lumbar puncture alone may not be sufficient. The collection of CSF from the lumbar cistern is more difficult and more likely to be contaminated with blood than that from the cerebellomedullary cistern (Chrisman, 1992). Sometimes no fluid or only a very small amount of fluid can be obtained owing to the small size of the lumbar subarachnoid space. Lumbar puncture may be preferred in cases with localized spinal disease because it may be more likely to confirm abnormality than cerebellomedullary cistern collections (Thomson et al., 1990).
Cerebrospinal Fluid Opening Pressure
CSF pressure is measured with a standard spinal fluid manometer as the fluid is collected (Simpson and Reed, 1987). CSF opening pressure should be measured to confirm a supposed increase in intracranial pressure due to a space-occupying mass or cerebral edema. Its normal range is less than 170 mmH2O (Lipsitz et al., 1999) and 100 mmH2O, for the dog and cat, respectively (Chrisman, 1991).
Handling of Cerebrospinal Fluid Specimens
Cells lyse rapidly in the low-protein milieu of CSF, so cell counts and cytologic preparations of unfixed fluid should be done within 30 to 60 minutes of collection (Fry et al., 2006). The likelihood of misinterpretation due to sample deterioration depends on the initial protein concentration of the sample and how long analysis is delayed. For example, if the CSF protein concentration is >50 mg/dl, a delay in analysis of <12 hours is unlikely to alter final interpretation. Addition of an equal volume of 4% to 10% neutral buffered formalin or 50% to 90% alcohol is recommended for fixation of specimens that cannot be immediately delivered to a laboratory and processed immediately (Carmichael, 1998). Alternatively, the addition of one drop of 10% formalin to 1 to 2 mL of CSF may be used to preserve cells for cell counts and morphologic examination when submitted to a referral laboratory, keeping in mind that cell counts will be affected but may not be clinically significant. Refrigeration will help retard cellular degeneration. Cellular stability can be increased by addition of fresh, frozen, or thawed serum or plasma (Bienzle et al., 2000) or by addition of 20% albumin (Fenner, 2000). If a CSF sample is not analyzed within 1 hour from collection, Fry and co-workers (2006) recommend to divide the fluid into 2 aliquots, an unadulterated aliquot for total nucleated cell count and protein measurement and an aliquot added with 20% of fetal calf serum (or 10% autologous serum) for differential cell count and morphologic evaluation. For those samples of insufficient volume (<0.5 mL total), hetastarch (1:1) can be added to CSF for all routine assays. In the last situation, the dilutional effect of adding a stabilizing agent must be taken into account when calculating results. Protein and enzyme concentrations in CSF are relatively stable, and submission to the laboratory by routine delivery, postal delivery, or courier is usually sufficient for accurate determinations (Carmichael, 1998).
Laboratory Analysis of CSF
Routine analysis of CSF is recommended in all cases in which it is collected; specialized analyses may be needed in selected cases. Routine analyses of CSF includes the following: macroscopic evaluation, quantitative analysis (erythrocyte count, nucleated cell count, and total protein), and microscopic evaluation as summarized in Table 14-1. If the volume of CSF is small and all tests are not likely to be obtained, the clinician should rank the tests in order of preference when the specimen is submitted to the laboratory. Rand (1995) indicates that the most useful diagnostic tests, in decreasing order, are nucleated and erythrocyte counts, sedimentation cytology, protein concentration, and cytocentrifuge cytology.
TABLE 14-1 Routine Evaluation of CSF
| Component of CSF Evaluation | Normal CSF | Abnormal CSF | Comments/Notes |
|---|---|---|---|
| Macroscopic Evaluation | |||
| Color | Colorless | Pink, red xanthochromic (yellow to yellow-orange). Occasional gray to green color may be seen. | Red or pink suggests blood; if due to intact erythrocytes, it will clear with centrifugation. Xanthochromia is an indication of previous hemorrhage with accumulation of oxyhemoglobin or methemoglobin from erythrocyte degradation; may occur with hyperbilirubinemia. May be graded as slight, moderate, or marked. |
| Turbidity | Clear, turbidity absent | Turbid or cloudy—slight, moderate, or marked | |
| Erythrocyte (RBC) count | Zero RBC considered normal, but frequently present in small numbers | Variable | Standard hemocytometer |
| Nucleated cell count | Variable | Standard hemocytometer | |
| Specific gravity | 1.004-1.006 | Most within reference interval for normal CSF | Of questionable value because only relatively marked increases in total protein result in changes that are detectable by specific gravity measurement |
| Total Protein (Microprotein) | |||
| Quantitation | Most commonly cited reference intervals indicate usually <30 mg/dl (cerebellomedullary) or <45 mg/dl (lumbar cistern) | Increased total protein seen in a variety of conditions | Microprotein method and reference values may vary with laboratory; use laboratory-established reference values. |
| Estimation | Most sensitive to albumin; detects ranges of protein that are useful for evaluation of most canine and feline CSF specimens; good correlation with standard dye-binding microprotein determinations. | ||
| Ames Multistix* (urine dipstick) | Trace to 1 + protein on urine dipstick is within normal limits | ||
| Microscopic Evaluation | |||
| Cell population | Lymphocytes and monocytoid cells predominate; very few mature, nondegenerated neutrophils may be present. A few erythrocytes may be seen. | Variable | |
* N-Multistix SG, Bayer, Miles, Diagnostic Division, Elkhart, IN.
Effect of Blood Contamination
Various formulas have been used to predict the effect of blood contamination on protein concentration and nucleated cell count in CSF (Parent and Rand, 1994; Rand et al, 1990). Rand (1995) indicates that red blood cell (RBC) counts greater than 30 cells/μl in CSF will have a profound effect on the total and differential cell counts. However, in a study (Hurtt and Smith, 1997) of iatrogenic blood contamination effects of total protein and nucleated cell counts in CSF, the RBC count was not significantly correlated with nucleated cell count or protein concentration in CSF from clinically normal dogs or those with neurologic disease. The study concluded that high CSF nucleated cell counts and protein concentrations are indicative of neurologic disease, even if samples contain up to 13,200 RBC/μl. Although blood contamination may make interpretation of CSF more difficult, red or pink CSF or CSF with a high RBC count should not be discarded as a useless specimen because cytologic evaluation may detect abnormalities (Chrisman, 1992).
Macroscopic Evaluation
Normal CSF is clear, colorless, and transparent and does not coagulate Deviations from normal should be recorded as part of the macroscopic evaluation and are often graded 1 to 4 + or as slight, moderate, or marked. Turbidity (Fig. 14-1A) is reported to be detectable if greater than 500 cells/μl are present (Fenner, 2000) or if at least 200 leukocytes/μl or 700 erythrocytes/μl are present (Parent and Rand, 1994).
Red to pink discoloration may be associated with iatrogenic contamination with blood or pathologic hemorrhage. Erythrophages or siderophages in a rapidly processed CSF specimen with fixative added immediately following collection support pathologic hemorrhage as an underlying cause. Xanthochromia is the yellow to yellow-orange discoloration (Fig. 14-1B) associated with pathologic hemorrhage due to trauma, vasculitis, severe inflammation, disc extrusion, or necrotic or erosive neoplasia. Occasionally xanthochromia will be seen with leptospirosis, cryptococcosis, toxoplasmosis, ischemic myelopathy, coagulopathy, or hyperbilirubinemia.
Quantitative Analysis
Cell Counts
To count nucleated cells, charge both chambers of the hemocytometer with undiluted CSF and place the unit in a humidified Petri dish for 15 minutes to allow cells to adhere to the glass. All nucleated cells are counted in the 10 large squares (four corner squares and one center square on each side) for a total nucleated cell count per microliter. Cell counts for erythrocytes are performed similarly. A study conducted to evaluate the usefulness of an automated cell counter in counting and differentiating cell types from canine CSF (Ruotsala et al., 2008) determined moderate correlation between this method and a hemocytometer for leukocyte values and excellent correlation for erythrocytes; however, cell differentials were much more variable. Results from this study also suggested that lymphocytes may be underestimated by manual microscopy in favor of large mononuclears.
Reference intervals for feline CSF erythrocyte counts are reported to range from 0 to 30 red blood cells per microliter (Parent and Rand, 1994). Reference intervals for feline CSF nucleated cell counts are reported to be less than 5 cells/μl, 0 to 2 cells/μl (Parent and Rand, 1994), less than 3 cells/μl (Chrisman, 1992), and less than 8 cells/μl (Cook and DeNicola, 1988).
Reference intervals for canine CSF erythrocyte counts are reported to be zero (Chrisman, 1992). Reference intervals for canine CSF nucleated cell counts are reported to be less than 5 cells/μl (Cook and DeNicola, 1988), and less than 6 cells/μl for cerebellomedullary cistern collections or less than 5 cells/μl for lumbar cistern collections (Chrisman, 1992).
Protein
Reference intervals for CSF total protein values may vary slightly with the laboratory and testing method used, but cerebellomedullary CSF protein is usually less than 25 to 30 mg/dl and lumbar cistern collections less than 45 mg/dl in dogs and cats (Chrisman, 1992; Fenner, 2000). Refractometer total protein evaluation is not accurate for assessment of CSF since the concentration of protein is quite low compared to serum or plasma and clinically significant changes may not be easily detectable on the refractometer scale. Special analytic techniques most often available at commercial or reference laboratories and not available in practice are needed owing to the minute protein concentration in CSF. Due to the minute amounts present, CSF protein analysis may be referred to as “microprotein.” An estimate of CSF protein content can be obtained using urine dipsticks. A membrane microconcentrator technique followed by agarose gel electrophoresis was recently described for measurement of cerebrospinal fluid proteins in dogs (Gama et al., 2007).
Other Tests
Other tests that have been recommended by various authors or used in specific situations include electrophoretic determination of albumin and determination of total immunoglobulin levels. In combination with the serum albumin level and serum immunoglobulin, these can be used to calculate the albumin quotient (AQ) and immunoglobulin G (IgG) index. The AQ is equal to the CSF albumin divided by serum albumin times 100. AQ greater than 2.35 suggests an altered blood-brain barrier with increased protein in CSF associated with leakage from plasma. The IgG index is equal to the (CSF IgG/serum IgG) divided by (CSF albumin/serum albumin). An IgG index greater than 0.272 with a normal AQ suggests intrathecal production of IgG. An increased IgG index and increased AQ are suggestive of an altered blood-brain barrier as the source of IgG (Chrisman, 1992).
Alterations in electrophoretic protein fractions have been reported to be useful in identifying inflammatory, degenerative, and neoplastic disease in combination with clinical signs. In general, dogs with canine distemper often have elevated CSF gamma globulins, most likely related to intrathecal production, while dogs with granulomatous meningoencephalitis (GME) may have elevated CSF beta and gamma globulins (Chrisman, 1992).
In a more recent work, Behr and co-workers (2006) found, using high-resolution protein electrophoresis, a strong linear correlation between CSF total protein concentration and AQ suggesting that an increased CSF total protein concentration is an indicator of blood-brain barrier dysfunction; moreover, although unexpected, the same authors found that electrophoretic profiles in a series of 94 dogs with different neurologic diseases were not characteristic of any particular disease concluding that high-resolution electrophoresis of paired CSF and serum samples cannot be considered a valuable ancillary diagnostic tool for canine neurologic diseases.
Detection of specific antibodies within the CSF and comparison with serum levels may be useful in diagnosis of infectious meningoencephalitides, including infectious canine hepatitis, canine herpesvirus, canine parvovirus, canine parainfluenza virus, canine distemper virus, ehrlichiosis, Rocky Mountain spotted fever, Lyme disease (borreliosis), Toxoplasma gondii, Neospora caninum, Encephalitozoon cuniculi infection, Babesia spp. infection, cryptococcosis (Berthelin et al., 1994), and blastomycosis. On the contrary, measurement of anticoronavirus IgG in CSF of cats with confirmed feline infectious peritonitis involving the central nervous system is considered of equivocal clinical use (Boettcher et al., 2007). Serial titers for serum IgG to show rising titer are helpful to demonstrate active disease. The presence of IgM in serum or CSF is considered more specific than IgG or total immunoglobulin levels for detection of active disease (Chrisman, 1992).
Glucose measurement in CSF and comparison with serum or plasma glucose levels are frequently cited. Normal CSF glucose is approximately 60% to 80% of the serum or plasma concentration (Fenner, 2000). However, changes in CSF glucose concentration in serum or plasma are not immediately reflected in CSF and may take 1 to 3 hours before they are apparent in CSF (Cook and DeNicola, 1988). The ratio between blood glucose and CSF glucose is frequently reduced in bacterial infections of the CNS in humans and has been reported to occur in some cases of pyogenic infections of the CNS, CSF hemorrhage, or blood contamination that may result in increased utilization of glucose by cells. However, the relationship between bacterial encephalitis and decreased CSF glucose compared with serum or plasma glucose may depend on multiple factors, including the blood glucose level, degree of permeability of the blood-brain barrier, and presence or absence of glycolytic cells or microorganisms. Fenner (2000) states that the reduction in glucose does not occur in dogs. Significant reductions in CSF glucose concentrations have been reported in human malignant disorders involving the leptomeninges and it is considered a relatively specific finding for this condition (Chamberlain, 1995).
Measurement of various electrolytes and enzymes in CSF has been reported. Their interpretation may be limited because of increases associated with altered blood-brain barrier permeability, concurrent evaluation of serum values, low benefit for cost, and poor correlation or specificity for particular pathologic processes or conditions (Chrisman, 1992; Cook and DeNicola, 1988; Parent and Rand, 1994).
Nevertheless, the CSF culture rarely assist in the identification of microorganisms: in a series of 8 confirmed cases of canine bacterial meningoencephalomyelitis, the CSF culture was positive in only 1 sample and the cultured bacteria was Corynebacterium spp. (Radaelli and Platt, 2002). Several factors likely contribute to poor culture performance in veterinary medicine —e.g., small volumes of CSF are collected for culture, the organisms are mostly confined to the brain parenchyma, some organisms are slow growing or require nonstandard culture techniques, and animal has received antibiotic therapy before sampling (Fenner, 1998).
The immunophenotype of cerebrospinal fluid mononuclear cells in the dog has been examined (Duque et al., 2002). According to authors, the main advantage of standardized immunophenotyping is having a more objective classification of mononuclear cells compared with the relative inconsistency of this classification when based solely on microscopic appearance.
Cytologic Evaluation of CSF
Cytologic evaluation of CSF is recommended for all collections as a valuable part of CSF evaluation. When necessary, clinicians are asked to rank tests in order of preference if less than 1-mL total volume is submitted. Usually, cell counts and total protein concentration are requested, followed by cytology. If additional CSF is available, other tests may be requested, depending on the differential diagnoses suggested by clinical signs, presentation, history, imaging studies, and results of cell counts, protein, and cytologic evaluation. Rand (1995) indicates that the most useful diagnostic tests in decreasing order are nucleated cell and erythrocyte counts, sedimentation cytology, protein concentration, and cytocentrifuge cytology.
Methods of Cytologic Preparation
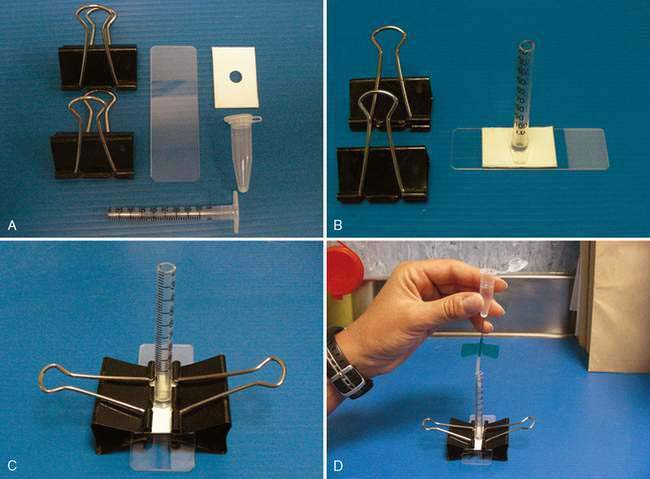
Cytocentrifugation is most commonly available in reference or commercial laboratories in which the volume of submissions justifies purchase of specialized equipment. The membrane filtration technique requires special staining that is not commonly available in practice, but which may be available at some reference or commercial laboratories. Several sedimentation techniques have been described and are suitable for use in practice or commercial or reference laboratories to which rapid submission of CSF specimens is possible (Cook and DeNicola, 1988; Parent and Rand, 1994). Readers are referred to these sources for more detail on construction of a sedimentation apparatus and preparation of sedimentation specimens. A sample device is demonstrated in Fig. 14-2A&B.
Cytocentrifuge or sedimentation preparations are most commonly air-dried and stained with Romanowsky stains that are commonly available in commercial or reference laboratories and clinical practice laboratories. Membrane-filtration specimens require wet-fixation and stains appropriate for this method, commonly Papanicolaou, Trichrome, or H & E. Wet-fixation and these staining methods may also be used on cytocentrifuge or sedimentation preparations and are appropriate for formalin- or alcohol-fixed specimens. Cytocentrifuge or membrane-filtration preparatory and staining techniques may vary with laboratory, technical training, and pathologist preference. Summaries of cytopreparatory and staining techniques for cytospin and membrane filtration specimens and specimens fixed in formalin or alcohol are available from a variety of sources. Interested readers are referred to Keebler and Facik (2008) as a recent comprehensive review.
Special stains may be indicated in some cases. Gram stain may be useful for confirmation and identification of categories of bacteria. India ink or new methylene blue preparations have been reported to be helpful in identification of fungal infections, especially cryptococcosis. Periodic acid-Schiff stain may be used to demonstrate positive intracellular material in dogs with globoid cell leukodystrophy. Luxol fast blue can be used to demonstrate myelin in CSF specimens (Mesher et al., 1996).
Cytologic Features of CSF
Several reviews of differential diagnoses and features of normal and abnormal cytology of canine and feline CSF with photomicrographs are available (Baker and Lumsden, 2000; Desnoyers et al., 2008). Cytologic features that may be found in canine and feline CSF are summarized in Table 14-2. Differential diagnoses associated with abnormal CSF findings are summarized in Table 14-3.
TABLE 14-2 Cytologic Features of Cerebrospinal Fluid in Dogs and Cats
| Cell or Feature | Description | Significance |
|---|---|---|
| Lymphocytes | Morphologically similar to those in peripheral blood; 9-15 μm in diameter, scant to moderate, pale basophilic cytoplasm with round to ovoid, slightly indented nucleus | Predominant cell type in normal CSF from healthy dogs; present in normal CSF from healthy cats |
| Reactive lymphocytes | Morphologically similar to those in peripheral blood; greater amount of cytoplasm and more deeply basophilic cytoplasm than normal lymphocytes; may see prominent perinuclear clear zones and coarse chromatin patterns | Not present in normal CSF from healthy animals, but not specific for underlying condition |
| Monocytoid cells | Large mononuclear cell; 12-15 μm diameter; moderate amount, pale basophilic, often finely foamy cytoplasm; nuclear shape variable to amoeboid; chromatin pattern open to lacy | Present in CSF from healthy animals in low numbers |
| Activated monocytoid cells | Morphologically resemble macrophages in many sites; larger than “normal” monocytoid cells (>12-15 μm diameter); increased amount of cytoplasm that is often paler than normal and possibly vacuolated; nuclei become round to oval and eccentric; chromatin with increased coarseness | Activation associated with irritation, inflammation, or degenerative processes; often phagocytic; reported in cats to be commonly associated with extensive necrosis |
| Neutrophils | Morphologically similar to those in peripheral blood; polymorphonuclear leukocytes | May be present in low numbers (up to 25% of total nucleated cells) in normal CSF from healthy animals |
| Ependymal lining cells | Uniform, round to cuboidal mononuclear cells; individual cells or in cohesive clusters; eccentric, round nuclei; uniformly granular to coarse chromatin; moderate amount of finely granular cytoplasm | May be present in normal CSF from healthy animals in low numbers; not consistently present in normal or abnormal conditions |
| Choroid plexus cells | Indistinguishable from ependymal lining cells (see above description) | May be present in normal CSF from healthy animals in low numbers; not consistently present in normal or abnormal conditions |
| Subarachnoid lining cells/leptomeningeal cells | Mononuclear cells with moderate to abundant pale basophilic cytoplasm; round to oval eccentric nuclei; uniform, delicate chromatin pattern; indistinct cytoplasm margins; single or in small clusters | May be present in normal CSF from healthy animals in low numbers; not consistently present in normal or abnormal conditions |
| Hematopoietic cells | Morphologically similar to those in bone marrow or other locations | Myeloid and erythroid precursors and erythroblastic island reported as contaminants of canine CSF with lumbar collections |
| Eosinophils | Morphologically similar to those in peripheral blood; polymorphonuclear leukocytes with eosinophilic granules with shape characteristic for species | Occasionally cells seen in normal CSF from healthy dogs or cats; may be seen as a nonspecific part of an active inflammatory response; also consider parasitic, hypersensitivity, or neoplastic processes (primary or metastatic) |
| Plasma cells | Morphologically similar to those in other locations; eccentric nuclei with prominent chromatin (“clockface” pattern); moderately abundant cytoplasm, moderately to deeply basophilic with perinuclear clear zone (Golgi apparatus) | Not present in normal CSF from healthy dogs or cats; may be part of nonspecific reactive or inflammatory process with response to antigenic stimulation |
| Bacteria | Morphology varies with type, may include cocci, rods of various sizes, coccobacilli, or filamentous forms | Not present in normal CSF from healthy dogs or cats; may be contaminants if collection process or tube are not sterile or if CSF collected close to death; pathologic role likely if suppurative meningitis is present and supported by intracellular location |
| Neural tissue | Nerve cells morphologically similar to those in nervous tissue; very large cell with prominent nucleolus, abundant cytoplasm, and three to four tentacle-like cytoplasmic processes; neuropil/myelin represented by amorphous, acellular background material | Reported as contaminant in canine CSF associated with accidental puncture of spinal cord; myelin fragments may be associated with demyelination |
| Paracellular coiled “ribbons” | Coiled, homogeneous, basophilic material within phagocytic vacuoles | Reported in CSF obtained at postmortem; hypothesized to represent denatured myelin, myelin figures, or myelin fragments |
| Neoplastic cells | Abnormal cell type or number for location (benign tumors) or atypical features fulfilling criteria for malignancy (malignant tumors); morphology may vary with cell type of origin and degree of differentiation | May be primary or metastatic; presence requires communication with subarachnoid space or ventricles; absence of tumor does not rule out its presence without contribution of cells to the CSF |
| Fungi/Yeast/Protozoa | Appearance varies with type; may be primary or opportunistic infections | Characteristic morphology associated with various common pathologic organisms; demonstration of organisms in conjunction with clinical signs and results of other testing increases confidence in diagnosis of fungal or protozoal disease |
| Mitotic Figures | Recognized by characteristic nuclear configurations of cells undergoing mitosis; cell type of origin not identifiable during the mitotic cycle | Rare mitotic figures reported in normal CSF from healthy animals; presence indicates proliferative process, often neoplasia |
TABLE 14-3 Differential Diagnoses Associated with Cytologic Features of Inflammation in the CSF
| Cytologic Features | Special Considerations or Differential Diagnoses | Comments |
|---|---|---|
Depends on species, type of infection, focal or diffuse involvement, presence of concurrent necrosis; presence of protozoa or fungi/yeast organisms or intracellular bacteria confirms diagnosis | ||
| Marked neutrophilic inflammation (suppurative meningitis) | Bacterial infection | May be focal (abscess) or diffuse (meningoencephalomyelitis); intracellular bacterial confirms diagnosis |
| Predominance of neutrophils (>50%), often with increased CSF protein | History may be supportive; may have traumatic, degenerative, metabolic infectious, neoplastic, or other underlying cause | |
| Mixed cell inflammation with a variety of cell types (no single cell type predominant) | Often interpreted to represent granulomatous inflammation—consider fungal, protozoal, parasitic, or rickettsial infection | Presence of fungal or protozoal organisms is confirmatory |
| Mixture of macrophages, lymphocytes, neutrophils, and sometimes plasma cells, with or without elevated CSF protein, with or without pleocytosis | Some idiopathic inflammatory or degenerative diseases Inadequately treated chronic bacterial infections or early response to antibacterial treatment | |
| Nonsuppurative inflammation (mononuclear pleocytosis) | Viral, bacterial, fungal, protozoal, parasitic, or rickettsial infection | Especially non-FIP viral meningoencephalomyelitis in cats and canine distemper infection in dogs |
| Pleocytosis with predominance of mononuclear cells, especially lymphocytes | Signalment and lymphocytic predominance helpful in diagnosis but definitive diagnosis requires histopathology; not responsive to glucocorticoids | |
| Eosinophilic inflammation | Parasitic, protozoal, bacterial, viral, or rickettsial infections | Uncommon manifestation reported with a variety of types of disease |
| Pleocytosis with predominance of eosinophils | Consider vaccine reactions or other hypersensitivity components associated with infectious or noninfectious origin |
Cytologic Features of Normal CSF
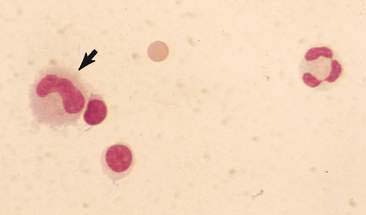
Normal CSF from healthy dogs and cats contains primarily mononuclear cells (Fig. 14-3) and is indicated to be a mixture of lymphocytes and large mononuclear (monocytoid) cells. The percentages of lymphocytes and monocytoid cells may vary with the method used for cytologic preparations, but lymphocytes are reported to be the predominant nucleated cell type in normal canine and feline CSF. However, Parent and Rand (1994) report monocytoid cells as the predominant type in normal CSF from healthy cats. They indicate monocytoid cells compose 69% to 100% of the nucleated cells, lymphocytes 0% to 27%, neutrophils 0% to 9% macrophages 0% to 3%, and eosinophils 0% to less than 1% of nucleated cells. Occasional neutrophils or eosinophils as within normal limits may be present as long as these cell types do not represent more than 10% or 1% of the nucleated cells, respectively. Low numbers of mature, nondegenerate neutrophils are occasionally seen in normal CSF from healthy dogs and cats and that rare eosinophils may be present. Occasional choroid plexus cells, ependymal cells, meningeal lining cells, or mitotic figures may be seen in normal CSF from dogs or cats (Chrisman, 1992; Rand, 1995).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree