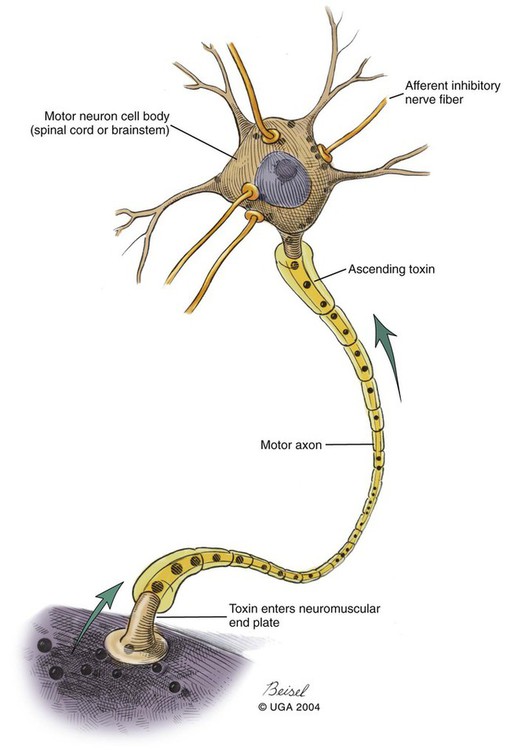
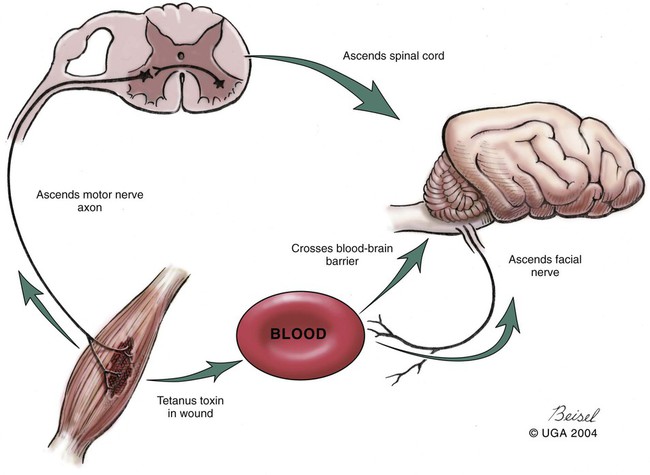
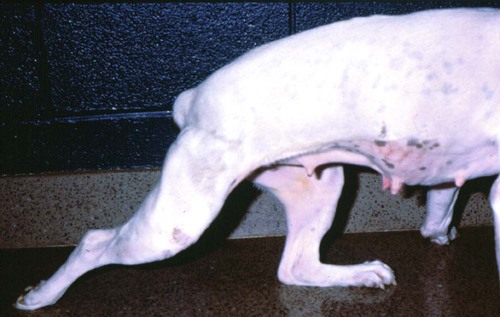
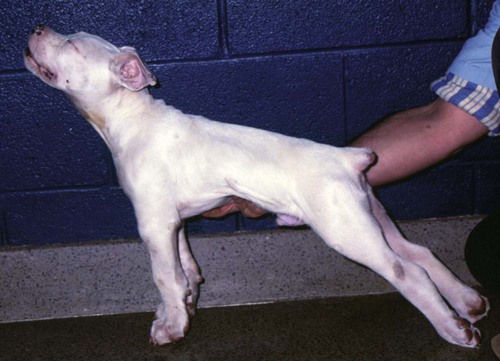
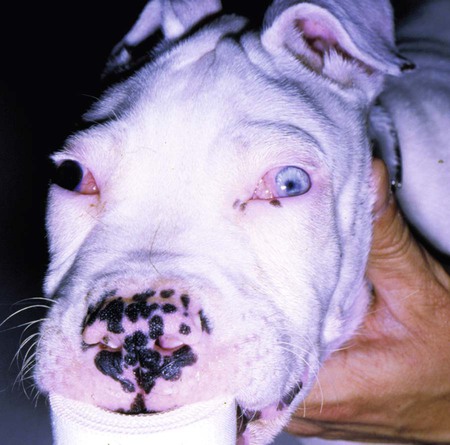
Tetanus is caused by the action of a potent neurotoxin formed in the body during the vegetative growth of Clostridium tetani. C. tetani is a motile, gram-positive, nonencapsulated, anaerobic, spore-forming bacillus. Although strain differences of C. tetani exist throughout the world, an antigenically homogeneous toxin is produced by all strains. Resistant spores of the organism can be found in the environment, especially in the soil, where increased moisture, cultivation, and fertilization favor their survival. Organisms are routinely isolated from the feces of many domestic animals, including the dog and cat.15 Isolation from human feces occurs with greater frequency in those occupationally exposed to farm animals. Spores can survive adverse weather conditions in the absence of direct sunlight for months or years and can be found readily in dust and debris in indoor environments. Spores are resistant to boiling water, phenol, cresol, and mercury bichloride, and they resist an autoclave temperature of 120° C for 15 to 20 minutes. However, the vegetative phase of C. tetani is no more resistant to chemical and physical inactivation than other microorganisms. The prevalence of the disease in dogs and cats is relatively low compared with that in other domestic animals, which may be related to the natural species resistance of dogs and cats to this toxin. The inherent resistance is related to the inability of the toxin to penetrate and bind to nervous tissue; direct central nervous system (CNS) injections of toxin produce the same signs in different species (Table 41-1). Tetanus also appears to be more severe in younger animals.7 This may relate to age-related natural immunity or increased environmental exposure with advancing age. TABLE 41-1 Relative Susceptibility of Animals to Tetanus Toxin Many experimental studies have been performed with dogs, cats, and other laboratory animals to elucidate the mechanism by which tetanospasmin enters and affects the CNS. The site and route of administration of toxin are important in determining the type of disease that develops. Localized tetanus can be produced by intramuscular or subcutaneous injection of toxin at a specific site. Tetanospasmin is a dimer composed of (1) a heavy chain (100 kDa) that binds to neuronal cells and transport proteins, and (2) a light chain (50 kDa) that blocks the release of neurotransmitters.34 Toxin enters the axons of the nearest motor nerves at the neuromuscular end plate and migrates by retrograde transport within motor axons at a rate of 75 to 250 mm per day to the neuronal cell body within the spinal cord (Fig. 41-1). Within the spinal cord, toxin ascends bilaterally until it reaches the brain (Fig. 41-2). The clinical signs of tetanus infection can be explained by the known pathophysiologic effects of tetanus toxin on the nervous system. It inhibits release of glycine and γ-aminobutyric acid (GABA)—neurotransmitters of inhibitory interneurons of the brain and spinal cord. Presynaptic blockade of synapses of Renshaw cells and 1a fibers of alpha motor neurons occurs. The binding of tetanus toxin to presynaptic sites of these inhibitory neurons is irreversible; recovery depends on sprouting of new axon terminals.34 Most experimental evidence has confirmed the effect of toxin at the spinal cord; however, the brain, neuromuscular junctions, and the autonomic nervous system can also be affected. Tetanus toxin has an affinity for gangliosides within the gray matter of the CNS, which may explain the cerebral signs that appear in some animals without obvious spinal cord involvement. The effects of tetanus toxin have also been ascribed to its affinity for binding at the neuromuscular junction, which may induce direct neuromuscular facilitation before the migration of toxin to the CNS. Tetanus toxin may affect sympathetic inhibitory neurons, in the same way it affects motor inhibitory neurons within the spinal cord, causing signs of autonomic dysfunction. Bradycardia associated with tetanus probably results from vagal-parasympathetic hyperactivity. Tetanus toxin also blocks neurotransmitters in the parasympathetic cardiac inhibitory center of the nucleus ambiguus, resulting in increased vagal tone and pronounced bradyarrhythmias. Increased catecholamine release associated with adrenergic stimulation can also cause episodes of hypertension or tachycardia in tetanus. Clinical signs of tetanus usually occur within 5 to 10 days after receiving a wound, although ranges of 3 to 18 days have been reported.25 This time varies and is shorter when the wound is closer to the CNS, has numerous organisms, and has a more anaerobic environment, which favors toxin production. Because of the increased resistance of cats and dogs to tetanus, disease onset may be delayed for up to 3 weeks. This delay may account for the absence of a detectable wound at the time of examination. However, because of greater innate resistance of cats as compared to dogs, the wound required to produce disease is so extensive that it is usually obvious. Wounds closer to the head (i.e., brain) are associated with more rapid onset and generalized CNS signs than injuries to distant extremities. In addition to external wounds, tetanus has also been frequently observed as a complication of foreign bodies, broken or deciduous teeth, postoperatively as with ovariohysterectomy, and after pregnancy or parturition, especially when associated with fetal death.3–5,23,25 Localized tetanus is more common than generalized tetanus in dogs and cats than it is in people and other domestic animals because of the relative resistance of carnivores to the toxin. Localized tetanus is more common in cats9 than dogs, presumably because of a greater resistance of cats to toxin. Increased stiffness of a muscle or an entire limb is first noted in close proximity to the wound site (Fig. 41-3). In the thoracic limb, the elbow is usually extended, and the carpus may be held flexed or extended. The stiffness usually spreads, gradually involving the opposite extremity. Although the rigidity may remain localized to both thoracic limbs and associated paraspinal regions,11 it usually spreads over a variable length of time and eventually involves the entire body.28 Localized tetanus in the pelvic limbs has been commonly associated with the female reproductive tracts of dogs and cats. In animals in which a single extremity is affected, the diagnosis may be unclear. Animals affected with generalized tetanus walk with a stiff gait and generally have an outstretched or a dorsally curved tail. They are not in pain but have difficulty in standing or lying down in comfortable positions because of the extreme muscle rigidity (Figs. 41-4 and 41-5). The rectal temperature is usually increased because of the excessive muscular activity. Postural reaction testing, such as a proprioceptive positioning evaluation, usually reveals normal initiation but stiff performance of the motor response. If the animal is extremely rigid, attempts to correct the knuckled limb may fail. When performing this type of testing, providing weight support is important for obtaining a more accurate evaluation. Myotactic reflexes are generally accentuated and flexor reflexes are depressed, but both may be difficult to elicit because of muscle stiffness. Intracranial signs develop in the late stages of localized tetanus. They begin earlier in animals with generalized tetanus and usually generalized muscular stiffness. Cranial nerve motor nuclei are affected, resulting in hypertonicity of respective musculature. Protrusion of the third eyelid and enophthalmos result from retraction of the globe, which is caused by extraocular muscle hypertonicity (Fig. 41-6). In addition, miosis is frequently present. The ears are held erect, the lips are drawn back (risus sardonicus), and the forehead is wrinkled as a result of facial muscle spasms. Trismus (“lockjaw”) is caused by excessive contraction of masticatory muscles. Other mechanical causes of trismus17 can be differentiated from tetanus, because they usually do not involve other body sites and the jaw cannot be opened during anesthesia. Increased salivation, increased heart and respiratory rates, laryngeal spasms, and dysphagia can result from involvement of parasympathetic and somatic cranial nerve nuclei. With laryngeal spasms, signs of labored inspiration may be prominent and phonation altered, although these signs may be compounded by the existing chest-wall muscle spasticity. Reflex muscle spasms occur in animals with generalized tetanus or intracranial involvement. Animals become apprehensive and react strongly to tactile or auditory stimulation. Mild stimulation may precipitate periodic generalized tonic contraction of all muscles with opisthotonos or cause grand mal convulsions. When tonic contractions begin to occur, the interval between spasms decreases until the animal reaches a convulsive state. Dogs and cats usually remain conscious until they develop convulsions. Reflex muscle spasms are painful, so animals may vocalize during such episodes. Animals with tetanus usually have a desire to eat, but because of jaw stiffness, they may have trouble prehending or swallowing solid food. Animals with complicating hiatal hernia begin regurgitating.2,7,13,40 Dysuria and urine retention, constipation, and gaseous distention are common results of persistent anal and urethral sphincter contractions. Urinary infections complicate the management of tetanus when indwelling catheters are used for recumbent animals. Decubital ulcers frequently develop in those animals that are recumbent for extended periods. Coxofemoral luxation, which has been observed in people with tetanus as a result of severe muscle contraction, has been reported in affected dogs.3,19 Hyperthermia is a consistent complication from constant muscle contractions or seizures. Nosocomial pneumonia is a common complication of tetanus and is caused by factors such as recumbency, reintubation or tracheostomy, aspiration, use of paralytic agents, or autonomic disturbances.8 The progression of clinical signs culminates in death, which is usually caused by respiratory compromise resulting from rigidity of the respiratory musculature, reflex spasms of the larynx, increased airway secretions, and central respiratory arrest from medullary intoxication or anoxia. Results of cardiac testing are frequently abnormal.5,7,7 Tachyarrhythmias and bradyarrhythmias may be noted in individuals with tetanus. Systemic hypertension with dramatic increases in systolic blood pressure are common. Rapid heart rates are usually associated with sinus or supraventricular tachycardia, and less commonly with ventricular tachycardia. Bradycardia (less than 70 beats/min) is characterized by atrioventricular heart block, sinus arrest or atrial standstill, and ventricular escape complexes. Fatality may result with either tachyarrhythmias resulting in ventricular fibrillation, or bradyarrhythmias resulting in cardiac arrest. The muscle spasms that develop in animals with tetanus are usually reduced but not always abolished by general anesthesia. Deep anesthesia in mildly affected animals may resolve all evidence of muscle hypertonicity. Even when clinically evident relaxation occurs, characteristic electromyographic changes can usually be detected. Insertion of the needle or tapping of the muscles or tendons is followed by persistent electrical motor unit discharges rather than the expected period of electrical silence. Continuous spontaneous motor unit discharge with simultaneous activity in both agonist and antagonist muscles is a characteristic finding.10,39 Muscle biopsy results are usually unremarkable in acute cases of tetanus, and any observed abnormalities may be attributed to muscle trauma caused by constant hypertonicity or prolonged recumbency. Mildly affected animals often respond well to treatment and have minimal hospitalization time. Therapy for tetanus in severely affected animals is costly and time consuming, and owners must be advised of the possibility of complications and a lengthy hospitalization. Time of hospitalization of severely affected animals can range from 7 to 40 days with a mean of approximately 20 days. Fortunately, the disease is often localized or mild in dogs and cats because of their innate resistance. However, untreated cases can prove fatal. In contrast, with successful recovery, there are no residual neurologic deficits. Because of their natural resistance, dogs and cats are not vaccinated with toxoid as a means of prophylaxis or treatment. Recommended dosages for the drugs used in the following treatment are summarized in Table 41-2. TABLE 41-2 Recommended Drug Dosages for Tetanus
Tetanus
Etiology
Epidemiology
Animal
Susceptibilitya
Horse
1 (most susceptible)
Guinea pig
2
Human
3
Mouse
12
Rabbit
24
Dog
600
Cat
7,200
Chicken
360,000 (least susceptible)
Pathogenesis
Clinical Findings
Clinical Complications
Diagnosis
Therapy
Druga
Species
Doseb
Route
Interval (hours)
Duration (days)
ANTIMICROBIALS
Penicillin G potassiumc
B
20,000–40,000 U/kg
IV, IM
6–8
10
Penicillin G procaine
B
20,000–40,000 U/kg
IM, SC
12–24
10
Amoxicillin-clavulanate
B
12 mg/kg
PO
12
10
Metronidazole
D
10–12 mg/kg
PO, IV
8
10
B
15 mg/kg
PO, IV
12
10
C
10–25 mg/kg
PO
24
10
Tetracycline
B
20–22 mg/kg
PO
8
10
Clindamycin
D
11–33 mg/kg
PO
12
10
C
11–33 mg/kg
PO
24
10
B
10 mg/kg
IV, SC, IM
12
10
IMMUNOTHERAPEUTIC AGENTS
Equine antitoxind
100–1000 U/kg
IV, IM, SC
Once
500-1000 U per site
Intralesional
Once
1–10 U/kg
Intrathecal
Once
Human tetanus immune globulin
B
500-2000 U near wound if found
IM
Once
SEDATIVES AND ANALGESICS
Acetylpromazine
B
0.01–0.07 mg/kge
IV
2–6
prn
B
0.1–0.25 mg/kge
IM
4
prn
B
1.0 mg/kg
PO
6–8
prn
Chlorpromazine
D
0.5 mg/kg
IM, SC
6–8
prn
C
0.2–0.4 mg/kg
IM, SC
6–8
prn
Midazolam
B
0.1–0.2 mg/kg
IM, IV
2–4
prnf
Diazepam
D
5.0–10 mg total
IV, PO, IM
2–4
prn
B
0.2–0.5 mg/kg
IV
4–6
prng
Pentobarbital
B
3–10 mg/kg
IV, IM
2–6
prnh
Phenobarbital
B
1–6 mg/kg
PO, IM
6–12
prni
Propofol
D
1–2 mg/kg
IV
Prn
prnj
Butorphanol
D
0.2–0.4 mg/kg
IV
4–6
prn
MUSCLE RELAXANTS
Methocarbamol
B
22–44 mg/kg, up to 130 mg/kg
PO, IV
8
prnk
Dantrolene
D
1–5 mg/kg
POl
8
prn
Magnesium
D
100 mg/kg
IV
24
prnm
AUTONOMIC AGENTS
Atropinen
B
0.05 mg/kg
SC
Prn
prn
Glycopyrrolaten
B
0.005–0.01 mg/kg
SC, IV
Prn
prn
1 mg total
PO
8
prn
Metoclopramide
B
0.28 mg/kg
POn
8
prn
SUPPLEMENT
Pyridoxine
B
100 mg total
PO
24
prn ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tetanus