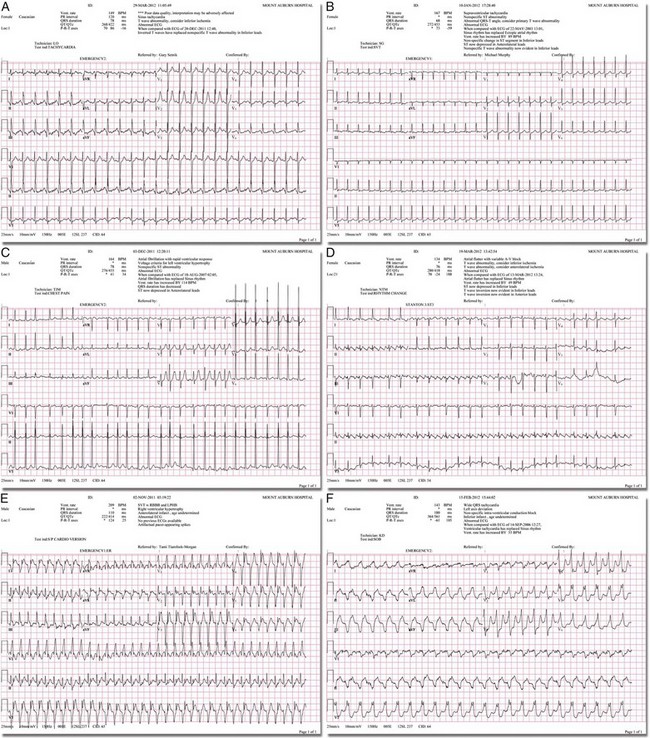
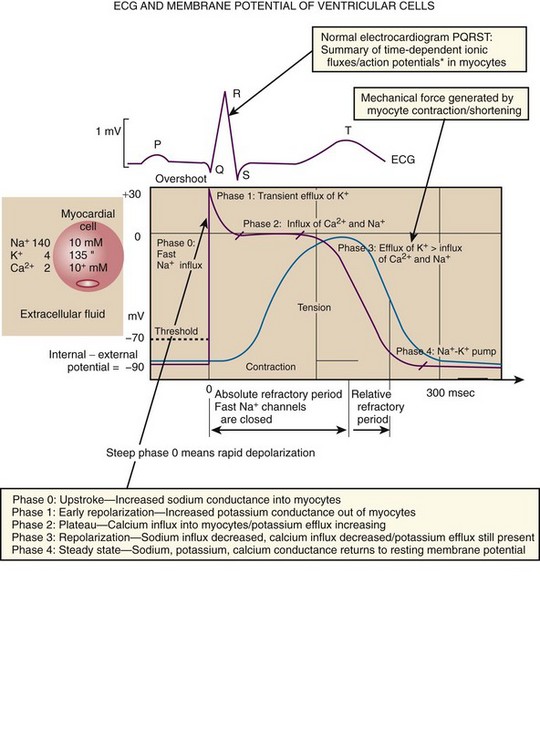
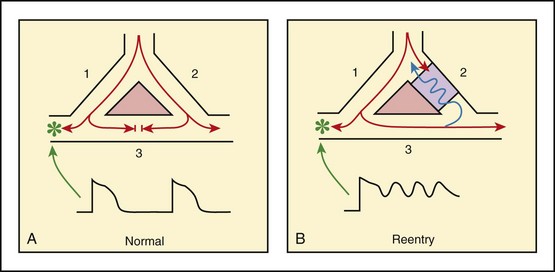
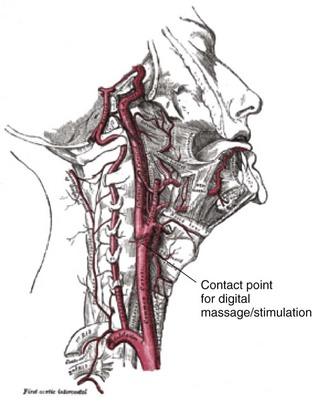
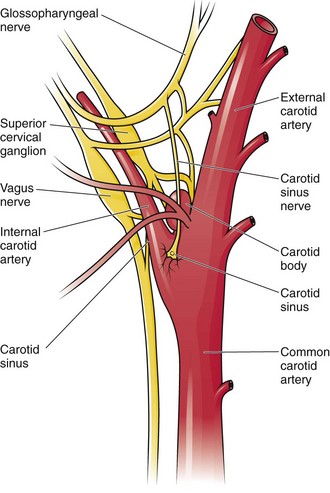
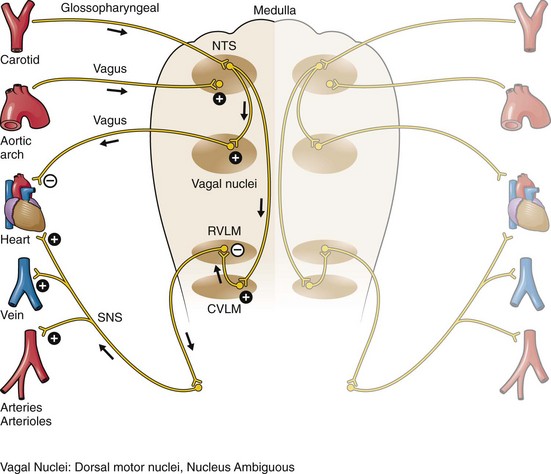
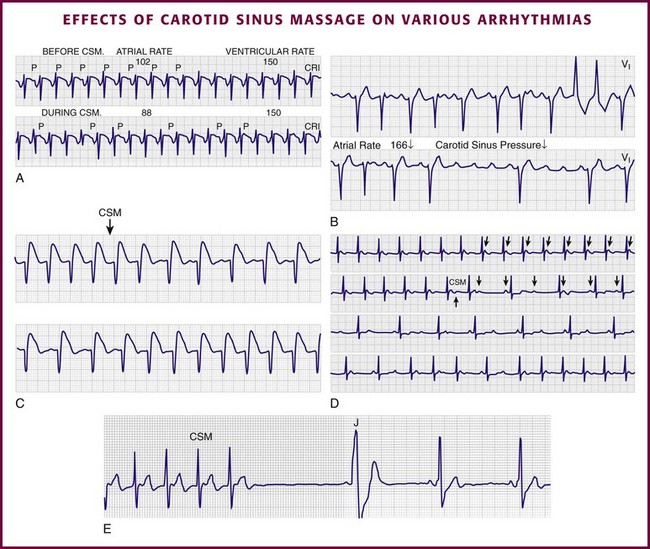
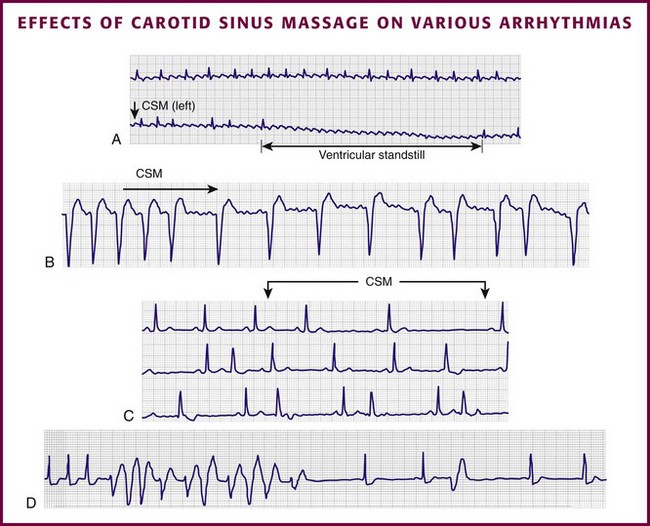
Chapter 11 Techniques for unmasking, identifying, and treating the various forms of tachyarrhythmias are presented in Box 11-1. This chapter addresses the utility of the vagal reflex in treating and managing various pathophysiologic conditions and the use of medications and cardioversion as they apply to the treatment of various supraventricular tachycardias (SVTs). The major focus is on the evaluation and treatment of SVTs. A more comprehensive discussion regarding the treatment of ventricular tachycardia (VT) is provided in Chapter 12. There are two general categories or types of tachycardias: SVT and VT. The term supraventricular tachycardia describes a rapid HR that has its electrochemical origin either in the atria or in the upper portions of the AV node. Ventricular tachycardias originate in the ventricular free walls or interventricular septum (or both). VTs can quickly become unstable and require special consideration (Fig. 11-1F). SVTs can be further classified as narrow-complex (QRS duration <0.12 second) and wide-complex tachycardias (QRS duration >0.12 second). The rhythms of these dysrhythmias can be regular or irregular. Examples of narrow-complex SVTs are sinus tachycardia (Fig. 11-1A); atrial fibrillation (AF) (Fig. 11-1C); atrial flutter (Fig. 11-1D); AV nodal reentry; atrial tachycardia (Fig. 11-1B), both ectopic and reentrant; multifocal atrial tachycardia (MAT); junctional tachycardia; and accessory pathway-mediated tachycardia. The term wide-complex tachycardia describes rhythms such as VT (Fig. 11-1F), SVT with aberrancy (Fig. 11-1E), or a preexcitation tachycardia facilitated by an accessory pathway between the atria and ventricles. Immediately thereafter, this depolarizing wave accelerates as it travels down the bundle of His to the Purkinje fibers and causes ventricular depolarization leading to contraction. Subsequently, the ventricles begin to relax (i.e., enter diastole and begin to fill with blood before the next depolarization). This describes the events of one cardiac cycle or heartbeat. The changes in electrochemical voltage during these events are depicted on the electrocardiogram in the usual sequential PQRST (the P wave indicates SA nodal depolarization, the PR interval denotes atrial depolarization followed by activation of the AV node, and the QRS complex summarizes electrical activity during ventricular depolarization) (Fig. 11-2). In addition to areas of increased automaticity that can precipitate SVTs, a condition described as reentry can also cause SVTs. Reentry describes a condition whereby a depolarization impulse is being propagated down a pathway in which some of the myocytes are still in the effective refractory period and a “unidirectional block” is present and preventing the impulse from traveling normally down this pathway. However, as the impulse travels around the area of the “unidirectional block,” the tissue allows the depolarization front to travel in the opposite (antidromic) direction, back to the initial point of entry into this pathway. This allows the depolarization wavefront to restimulate the myocytes and initiate another propagated depolarization through the same tract (Fig. 11-3). If this condition persists and these impulses stimulate the atria effectively and traverse the AV node, an SVT may develop as a result of reentry. Suppression of this dysrhythmia can be achieved by terminating the conditions favoring reentry, and the hemodynamic consequences may be attenuated by enhancing AV nodal blockade of the ventricles (e.g., through vagal stimulation, medication), thus slowing the ventricular response to this condition. Termination of reentry can be accomplished by either pharmacologic modification of the myocytes to render them refractory to depolarization impulses for a longer period in a stable patient or by synchronized cardioversion to uniformly depolarize the myocytes and terminate the conditions favoring the SVT. To complete this discussion, we must also consider that there may be the possibility of an interventricular conduction delay being present before the development of an SVT. If this is the case, the SVT may appear as a wide-complex tachycardia and can be confused with other dysrhythmias. However, an even more dangerous situation can occur if a wide-complex tachycardia of ventricular origin (VT) is present and is misdiagnosed as an SVT with aberrancy. As a result, the patient could be treated inappropriately, with the intervention causing suppression of ventricular activity and ultimately cardiac arrest. VT with a pulse is considered an unstable rhythm that often requires synchronized cardioversion (discussed in more detail in Chapter 12). The physiologic effects of pressure on the carotid sinus have been known for centuries. They were first described in the medical literature in 1799, when Parry wrote a treatise titled “An Inquiry into Symptoms and Causes of Syncope Anginosa, Commonly Called Angina Pectoris.”1 He noted that pressure on the bifurcation of the carotid artery produced dizziness and slowing of the heart. The term carotid is derived from the Greek karos, which means heavy sleep. The bifurcation of the common carotid artery possesses an abundant supply of sensory nerve endings located within the adventitia of the vessel wall (Figs. 11-e1 and 11-e2). These nerves have a characteristic spiral configuration; they continually intertwine along their course and eventually unite to form the carotid sinus nerve. The afferent impulses travel from the carotid sinus via Herring’s nerve or the carotid sinus nerve to the glossopharyngeal nerve (CN IX) and then to the vasomotor center in the medullary area (nucleus tractus solitarius) of the brainstem (Fig. 11-e3). The vasomotor center is composed of three distinct areas, each with a distinctive function. The vasomotor center is located bilaterally in the reticular substance of the medulla and in the lower third of the pons. The center transmits efferent impulses downward through the spinal cord and the vagus nerve. The efferent impulses, which originate in the medial portion of the vasomotor center, travel along the vagus nerve (CN X) to the sinus node and the AV node of the heart. The vasomotor center’s medial portion lies in immediate apposition to the dorsal motor nucleus of the vagus nerve (CN X). These impulses in the medial portion of the vasomotor center decrease HRs. Efferent impulses originating in the lateral areas of the vasomotor center travel along the sympathetic chain to the heart and to the peripheral vasculature. These sympathetic impulses control either vasoconstriction or vasodilation of the vascular system. A balance between vasoconstriction and the vasodilation maintains proper vasomotor tone.2,3 The afferent nerve endings in the carotid sinus are sensitive to MABP and to the rate of change in pressure. Research indicates that pulsatile stimuli are more effective than sustained pressure in evoking a response. Elevated blood pressure stretches the baroreceptors, which leads to increased firing of the afferent nerve endings.2 As for low–blood pressure states, the carotid sinus baroreceptors are exquisitely sensitive to low blood pressure. Hypotension causes a drop in afferent firing.2 The parasympathetic and sympathetic nervous systems play independent but coordinated roles in the carotid sinus reflex. Increased firing of the carotid sinus results in reflex stimulation of vagal activity and reflex inhibition of sympathetic output. The parasympathetic effect is almost immediate; it occurs within the first second and causes a drop in HR. The sympathetic effect, which causes a drop in blood pressure through vasodilation, becomes manifested only after several seconds.4 The changes in blood pressure may not take full effect until a minute has elapsed.5 The changes in blood pressure and HR are independent phenomena. Epinephrine blocks the reduction in blood pressure, whereas a fall in HR is blocked by the administration of atropine. The parasympathetic branch of the carotid sinus reflex supplies the sinus node and the AV node. The effect of parasympathetic stimulation is to slow the HR. The SA pacemaker is more likely to be affected than the AV node, except when digitalis has been administered.2,5,6 Vagal maneuvers are potentially useful in attempting to slow down or break an SVT. They are also indicated in settings in which slowing conduction in the SA or AV node could provide useful information (Box 11-2 and Figs. 11-4A-E and 11-5A-D). Such settings include patients with wide-complex tachycardia, in whom carotid sinus massage (CSM) aids in the distinction between SVT and VT. CSM can elucidate narrow-complex tachycardia in which the P waves are not visible or aid in detection of suspected rate-related bundle branch block or pacemaker malfunction. After CSM, a wide-complex SVT may be converted to normal sinus rhythm, P waves may be revealed after increased AV node inhibition, or ventricular complexes may narrow as the ventricular rate slows. Because CSM slows atrial and not ventricular activity, AV dissociation may be seen more easily and is indicative of VT (see Fig. 11-4). In rapid AF or atrial flutter with a 2 : 1 block, either P waves or irregular ventricular activity with absent P waves may be revealed (Figs. 11-5A and B). Sinus tachycardia may also be more apparent once P waves are unmasked by slowing the SA node (see Figs. 11-4C and D). Adenosine may be used for the same diagnostic purpose in these situations as well.7 In order of decreasing frequency, the electrocardiographic changes seen with CSM and vagal maneuvers are presented in Box 11-3. Vagal maneuvers, CSM in particular, may also be a useful aid to the diagnosis of syncope in the elderly. Some 14% to 45% of elderly patients referred for syncope are thought to have carotid sinus syndrome (CSS).6,8,9 CSS is defined as an asystolic pause longer than 3 seconds or a reduction in systolic blood pressure greater than 50 mm Hg in response to CSM (Fig. 11-6). Because it shares many characteristics with sick sinus syndrome, it has been suggested that both are manifestations of the same disease. CSS causes cerebral hypoperfusion, which can lead to dizziness and syncope. Analysis of patients with CSS indicates that it results from baroreflex-mediated bradycardia in 29%, hypotension in 37%, or both in 34%.10,11 Therefore, syncope, chronic near-syncope, or a fall of unclear etiology in the elderly is an important indication for diagnostic CSM.12,13 Although the use of digoxin has been overshadowed by the use of other potentially less toxic agents such as calcium channel blockers and β-blockers, the clinician can still prospectively simulate the cardioinhibitory effects of digoxin on a patient by performing vagal maneuvers. This can guide use and dosage of the medication before initiating treatment with digoxin. Significant slowing or block with CSM suggests a similar sensitivity to digoxin, and a smaller loading dose should be considered (Table 11-1). TABLE 11-1 Ventricular Response to Carotid Sinus Massage and Other Vagal Maneuvers Adapted from Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia: Saunders; 2001:642.
Techniques for Supraventricular Tachycardias
Introduction
Overview and Significance: Anatomy and Physiology of Supraventricular Tachycardia
Vagal Maneuvers
Background Anatomy and Physiology
Indications for Vagal Maneuvers
TYPE OF ARRHYTHMIA
ATRIAL RATE (bpm)
RESPONSE TO CAROTID SINUS MASSAGE AND RELEASE
Normal sinus rhythm
60-100
Slowing with return to the former rate on release
Normal sinus bradycardia
<60
Slowing with return to the former rate on release
Normal sinus tachycardia
>100-180
Slowing with return to the former rate on release; appearance of diagnostic P waves
AV nodal reentry
150-250
Termination or no effect
Atrial flutter
250-350
Slowing with return to the former rate on release; increasing AV block; flutter persists
Atrial fibrillation
400-600
Slowing with persistence of a gross irregular rate on release; increasing AV block
Atrial tachycardia with block
150-250
Abrupt slowing with return to a normal sinus rhythm on release; tachycardia often persists
AV junctional rhythm
40-100
None; ± slowing
Reciprocal tachycardia using accessory (WPW) pathways
150-250
Abrupt slowing; termination or no effect; may unmask WPW
Nonparoxysmal AV junctional tachycardia
60-100
None; ± slowing
Ventricular tachycardia
60-100
None; may unmask AV dissociation
Atrial idioventricular rhythm
60-100
None
Ventricular flutter
60-100
None
Ventricular fibrillation
60-100
None
First-degree AV block
60-100
Gradual slowing caused by sinus slowing; return to the former rate on release
Second-degree AV block (I)
60-100
Sinus slows with an increase in block; return to the former rate on release
Second-degree AV block (II)
60-100
Slowing
Third-degree AV block
60-100
None
Right bundle branch block
60-100
Slowing with return to the former rate on release
Left bundle branch block
60-100
Slowing with return to the former rate on release
Digitalis toxicity–induced arrhythmias
Variable
Do not attempt CSM
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Techniques for Supraventricular Tachycardias