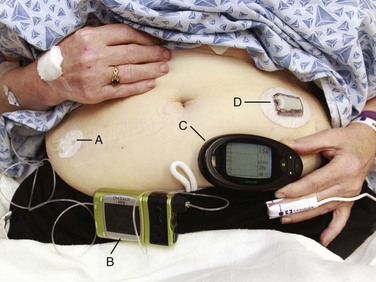
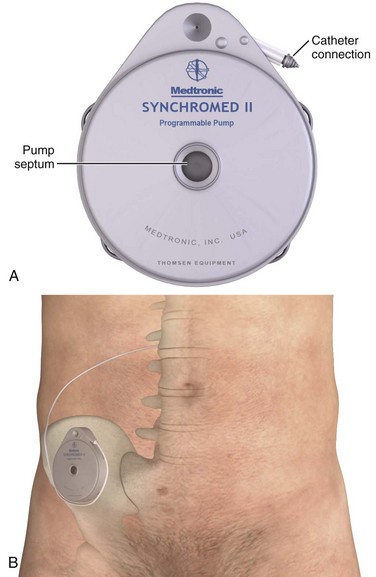
Chapter 71 In addition to cardiac pacemakers and defibrillators, a number of noncardiac devices have been developed for electronic neuromodulation and drug delivery.1,2 Although these devices are placed by a variety of subspecialists for the treatment of chronic illnesses, if the devices malfunction, patients may arrive at the emergency department in an acute state, thereby necessitating intervention by the emergency physician. External insulin infusion devices are becoming increasingly popular since their introduction in 1974. As of 2007, more than 375,000 external insulin infusion pumps are in use.3 An external insulin infusion pump device consists of a portable, programmable infusion pump connected to a subcutaneously implanted catheter maintained in place with adhesive tape (Fig. 71-1). The implanted catheter site varies, but it is commonly placed on the abdomen in adults and on the buttocks in young children. The thighs, hips, and upper part of the arms are other sites. It is recommended that the implanted catheter be replaced every 2 to 3 days. In 2010, the Food and Drug Administration (FDA) published a panel report that highlighted problems associated with insulin infusion devices.3 From the top five device manufacturers, the FDA noted 16,640 adverse events, including 310 deaths, 12,093 injuries, and 4,294 malfunctions. Box 71-1 lists the most frequently reported problems with devices in descending frequency. Box 71-2 lists the most frequently reported patient-oriented adverse reactions, which include hyperglycemia and hospitalization. Intrathecal drug delivery systems (IDDSs) have been in clinical use since the 1980s. Although data are accumulating, very few robust clinical studies of IDDSs have been published.4 The FDA approved the use of intrathecal baclofen in 1992 for severe spasticity secondary to spinal cord injury, multiple sclerosis, cerebral palsy, or stroke. In addition, the FDA approved the use of intrathecal morphine in 1995 for chronic pain refractory to traditional medical therapies. An IDDS is also used for the delivery of chemotherapeutic medications for specific oncologic conditions. Moreover, there are numerous off-label uses of various intrathecal medications. Currently, Medtronic is the only IDDS manufacturer in the United States. Medtronic’s SynchroMed II Programmable Infusion Pump consists of an infusion pump connected to an intrathecal catheter (Fig. 71-2A). The infusion pump is available with a refillable reservoir of either 20 or 40 mL. The pump is powered by a permanent lithium battery that cannot be recharged. The battery must be surgically replaced every 4 to 7 years. Normal refill intervals are usually between 2 and 3 months. The pump is placed in a subcutaneous pocket, generally in the right lower abdominal region. The intrathecal catheter is inserted slightly lateral to the spinous process into an appropriate lower lumbar interspace and connects to the pump via a subcutaneous tract that wraps around the abdominal wall (see Fig. 71-2B). Several studies have shown a significant rate of adverse effects in IDDS patients ranging anywhere from 2% to 50%.5,6 Medications currently in use via IDDSs include clonidine, bupivacaine, morphine, hydromorphone, fentanyl, ziconotide, and baclofen.7 Of these, bupivacaine and morphine have specific antidotes (Intralipid and naloxone, respectively). Medication overdoses, aside from standard emergency management, can also be treated by accessing the infusion pump reservoir. A unique complication related to IDDSs that was identified by the FDA and issued as a warning is the formation of a granuloma at the tip of the intrathecal catheter. This can obstruct infusion of the medication and lead to withdrawal symptoms.8,9 With regard to intrathecal opioids, the degree of lipophilicity of the intrathecal opioid affects its pharmacokinetics. Fentanyl, with its high lipophilicity, is absorbed rapidly by the spinal cord and little is left to ascend in cerebrospinal fluid (CSF). Thus, it has a rapid onset and shorter duration with fewer adverse effects. Morphine, with its high hydrophilicity, penetrates the spinal cord slowly, which allows a considerable amount of the drug to ascend in CSF. This, in turn, results in a slower onset and longer duration with a higher incidence of adverse effects, including pruritus, nausea and vomiting, urinary retention, changes in mental status, and respiratory depression.10,11
Noncardiac Implantable Devices
Insulin Infusion Devices
Anatomy
Device Complications
Intrathecal Drug Delivery Systems
Anatomy
Device Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine