CHAPTER 12 Synovial Fluid Analysis
ARTHROCENTESIS
In the verification, localization, diagnosis, and management of arthritis, synovial fluid examination is a key component of an initial medical database that includes clinical history, physical examination, radiographs, complete blood count, biochemical profile, and urinalysis. See Boxes 12-1 and 12-2 for indications and contraindications for arthrocentesis.
EQUIPMENT
Sterile disposable 3-ml syringes and 1-inch, 22-gauge or 25-gauge (small dogs and cats), hypodermic needle are recommended. In large breed dogs sampling of the elbow or shoulder joints may require a 1½-inch needle and the hip joint may necessitate a 3-inch spinal needle. Microscope glass slides with frosted ends, red-top tubes, and ethylene-tetra-acetic acid (EDTA) blood tubes should be readied and labeled with the patient’s name and the joint sampled. See Box 12-3 for a complete list of materials.
SAMPLE HANDLING AND TEST PRIORITIES
Laboratory tests performed can be limited by volume of synovial fluid collected. While the sample is in the syringe, volume, color, and turbidity should be noted. Viscosity is then assessed as the sample is expelled onto a glass slide for direct smears. Direct smears are immediately made for subsequent cytologic examination, nucleated cell differential count, and subjective assessment of cellularity. See Tables 12-1 and 12-2 for specific volumes needed and sequence of testing. When larger volumes of fluid are collected, a total nucleated cell count, mucin clot test, and total protein estimation, in order of priority, can be added to the aforementioned procedures.
TABLE 12-1 Test Priorities for 2 ml or More Synovial Fluid
| Amount | Test | |
|---|---|---|
| 1 drop | Cytology and WBC differential with viscosity estimate | Glass slide |
| 0.5 to 1.0 ml | Total nucleated cell count | Lavender top or plain |
| 1 to 3 ml | Bacterial culture and sensitivity | 20-ml (pediatric) BD BBL Septi-Chek blood culture tube |
| or | or | or |
| 0.5 to 1.0 ml | Bacterial culture and sensitivity | Sterile plain blood tube |
TABLE 12-2 Test Priorities for Less than 1 ml of Synovial Fluid
| Amount | Test | |
|---|---|---|
| 1 drop | Cytology and WBC differential with total nucleated cell estimate and viscosity | Glass slide |
| 2 or 3 drops | Bacterial culture and sensitivity | Culturette |
Cells in sediment smears and direct smears of fluid with the normally high viscosity may not spread out well on slides, making cell identification and differential cell count difficult. If this problem is encountered, it can be overcome by mixing an equal volume of hyaluronidase at 150 IU/ml with the synovial fluid and incubating for at least 10 minutes.1 The result is a fluid that facilitates better presentation of cell morphology and more complete cytologic evaluation.
LABORATORY ANALYSIS AND REFERENCE VALUES
Volume
An approximate or subjective estimation of fluid volume collected should be recorded.
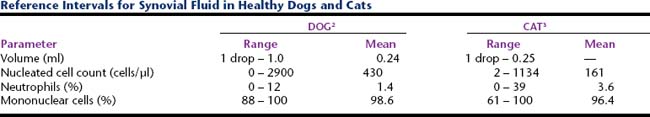
Synovial fluid volumes depend on patient size and which joint is being collected (within an individual there is variation from joint to joint). In normal animals, fluid volume can range from 1 drop to 1.0 ml in dogs and 1 drop to 0.25 ml in cats.1–3 Clinical experience is an extremely valuable guide to detecting an articular effusion. This judgment is based on the degree of joint capsule distension, ease of fluid collection, and volume readily obtained. The aim of arthrocentesis for synovial fluid analysis is to collect some synovial fluid, but not to drain the joint space.
Viscosity
Viscosity is easily assessed at the time of collection. However, if it must be evaluated after the sample is added to an anticoagulant, heparin is probably preferable to EDTA for sample preservation. EDTA tends to degrade hyaluronic acid and may decrease the sample’s viscosity.4
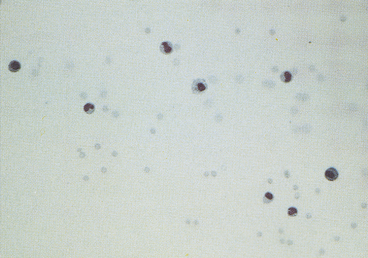
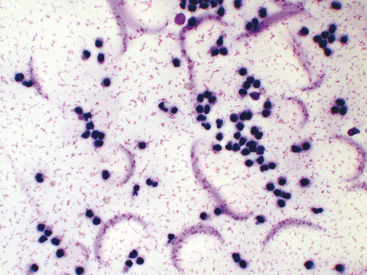
Viscosity can also be subjectively assessed when cytologically evaluating direct or sediment smears such that smears of fluids with normally high viscosity tend to have cells aligned in a linear pattern that is sometimes referred to as windrowing (Figure 12-1). In contrast, synovial fluid samples with decreased viscosity have cells more randomly arranged on the smear (Figure 12-2).
Mucin Quality
If sufficient sample remains following slide preparation and nucleated cell count, synovial fluid mucin quality or hyaluronic acid may be assessed using a mucin clot test. When there is the potential for clotting of joint fluid, heparin is recommended as an anticoagulant because EDTA interferes with the mucin clot test by degrading hyaluronic acid.4
Total Cell Counts
Nucleated cell counts in normal synovial fluid vary from joint to joint within an individual animal.1 However, surveys have not shown these differences to be either statistically significant or clinically relevant. Various canine reference intervals have been reported (Table 12-3). As a generalization from these studies, most normal joints have nucleated cell counts <3000 cells/μl.
TABLE 12-3 Reference Intervals for Synovial Fluid Total Nucleated Cell Counts in Healthy Dogs
| Range (cells/μl) | Joints sampled |
|---|---|
| 33-24951 | 12 joints: stifle, shoulder, carpus |
| 0-29002 | 55 joints: hip, stifle, hock, elbow, shoulder, carpus |
| 700-440010 | 20 stifles |
| 327-145011 | 14 stifles |
| 50-272512 | 58 stifles |
| 209-20705 | 19 stifles |
A recent study of synovial fluid samples from clinically normal cats, showed white blood cell (WBC) counts of 161 ± 209 cells/μl (mean ± SD) and median WBC of 91 cells/μl with a range of 2 to 1134 cells/μl.3 Samples were excluded from this study when there was gross evidence of blood contamination, radiographic evidence of osteoarthritis, or histologic evidence of synovitis or if postmortem physical examination revealed abnormalities. As a generalization from this study, most normal joints have nucleated cell counts <1000 cells/μl (Table 12-4).
A comparison of manual hemacytometer and electronic, automatic, particle counting of nucleated cells in canine synovial fluid revealed that the mean electronic total nucleated cell count was statistically higher than the mean manual count.5 Manual counting methods can demonstrate within-day and between-day analytical imprecision that is statistically higher than that of automated particle counting instruments.6,7 In general, differences in mean cell counts and precision have not proven to be clinically relevant; therefore, the efficiency and speed of automatic particle counters offer an advantage over manual methods.
EDTA is preferred as an anticoagulant and preservative for cytologic examination and nucleated cell counts. In comparison of EDTA versus heparin anticoagulants as a preservative for synovial fluid, samples stored in heparin showed a 4-fold and 9-fold greater decrease in total nucleated cell counts over 24 hours and 48 hours at 4°C, respectively.6 On the other hand, EDTA reportedly decreases synovial fluid mucin quality; therefore, total nucleated cell counts on fluid samples collected into EDTA may not be increased by hyaluronidase. Regardless of the anticoagulant/preservative used, synovial fluid should be pretreated with hyaluronidase when automated hematology analyzers are used for cell counts.8,9
Total Protein Concentration
Comparatively few studies have reported baseline values for total protein concentration, which probably reflects the relatively low priority given to this value. Other tests are preferred because sample volume is usually insufficient to allow for protein measurement. Synovial fluid protein concentration is best measured by a quantitative biochemical assay because refractometry measures other solutes and protein. A study of normal stifle, shoulder, and carpal joints reported a total protein concentration reference interval of 1.8 to 4.8 g/dl, as measured by refractometer.1 Normal synovial fluid does not clot in vitro because it is essentially free of fibrinogen and other clotting factors. Joint fluid can form a thixolabile gel if left undisturbed for several hours. Because clots are not thixotropic, normal fluid is distinguishable from clotting by gently shaking the sample to restore fluidity. If a specimen forms a clot after collection, this indicates intra-articular hemorrhage or inflammation with increased vascular permeability and protein exudation into the joint space.
CYTOLOGIC EXAMINATION
Normal synovial fluid contains very few RBCs. Increased RBC numbers may result from hemorrhage associated with collection and trauma or inflammation involving the joint capsule. Generalizations regarding the cellularity of synovial fluid can be consistently made via freshly prepared direct smears. The body of the smear of normal specimens contains about 2 cells/field at 400× magnification (40× objective). Cellularity of direct smears are categorized as normal (see Figure 12-2), slightly increased, moderately increased (Figure 12-3), or markedly increased (see Figure 12-1). Due to unpredictable variation among processing techniques and instrumentation, such assessments are impractical with concentrated specimens (sediment and cytocentrifuge smears).
Because of the high viscosity of normal synovial fluid, cells of direct and centrifuged sediment smears tend to line up in rows (i.e., windrowing, see Figure 12-1). This characteristic arrangement can be used to comment on sample viscosity, when volume is not sufficient for viscosity and mucin clot tests. However, in smears from synovial fluid with low cell counts, windrowing may not be apparent, even though viscosity is normal.
Smears of normal, and sometimes abnormal, synovial fluid can have a pink granular proteinaceous background (see Figures 12-3 and 12-13) that must not be confused with bacteria.
Nucleated cells should be classified as neutrophils, large mononuclear cells, lymphocytes, or eosinophils. Classification as mononuclear cells encompasses those that are phagocytically active. These cells could be derived from blood monocytes, tissues macrophages, or synovial lining cells. The origin of these cells has little practical importance regarding clinical diagnosis and therapy. The proportion of large mononuclear cells that have phagocytized debris, cells, or microorganisms should be recorded. On smears that are freshly made or from fluid not exposed to EDTA, the degree of vacuolated large mononuclear cells should be noted and reported as mild, moderate, or marked. Overall assessment of nucleated cell morphology ought to include comments on the degree of karyolysis, pyknosis, and karyorrhexis. Delayed processing can lead to nuclear degeneration and increased numbers of markedly vacuolated large mononuclear cells.1 Synovial fluid nucleated cell differentials are reported as percentage values and are incorporated into the interpretation of a total nucleated cell count or subjective assessment of cellularity.
Reports of normal canine synovial fluid nucleated cell differentials indicate neutrophils can make up as much as 12% of nucleated cells, but frequently compose < 5% of all nucleated cells.1,2,10,11 Eosinophils are absent.1,2,10–12 Lymphocyte values can be quite variable, with studies reporting 0% to 100% (mean 44%) and 3% to 28% (mean 11%).1,2 The balance of nucleated cells in normal joints are large mononuclear cells, ranging from 64% to 97% in one study, to 60% to 92% in another study. In samples of normal joints processed immediately by direct smear, the percentage of large mononuclear cells that were markedly vacuolated was about 9%.1 As with delayed processing, pre-treatment of fluid with hyaluronidase can increased the percentage of large mononuclear cells that are markedly vacuolated to about 14% to 18%.
A study of normal feline synovial fluids reports that mononuclear cells predominate, ranging from 61% to 100% of all nucleated cells (mean 96.4%), with smaller proportions of neutrophils that vary from 0% to 39% of all nucleated cells (mean 3.6%).3 Within the mononuclear cell component, lymphocytes or small mononuclear cells make up 0% to 45% of all mononuclear cells (mean 9.1%) and large mononuclear cells include 0% to 100% of all mononuclear cells (mean 81%). Freshly prepared smears from these cats reveal that among mononuclear cells, 0% to 100% are vacuolated (mean 9.9%).
BACTERIOLOGIC CULTURE
Conventional agar-based or broth-type bacteriologic culture methods have been shown to lack sensitivity when compared with blood culture methods.13–15 Liquid blood culture media offers several advantages, including culture of larger volumes compared with culture plates, resins that decrease the inhibitory affects of antibiotics and substances intrinsic to synovial fluid, and lytic agents that release microorganisms phagocytized by inflammatory cells. Because relatively small volumes are retrieved with arthrocentesis of cats and dogs, pediatric blood culture bottles or tubes are most appropriate. Blood culture tubes or bottles should be inoculated at the time of fluid collection and incubated for 24 hours at 37°C, at which time the result is transferred to appropriate growth media.
It has been suggested that culturing a synovial membrane biopsy specimen may be superior to culture of synovial fluid, but this was not shown to be the case when stifle joints were experimentally infected with Staphylococcus intermedius.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree