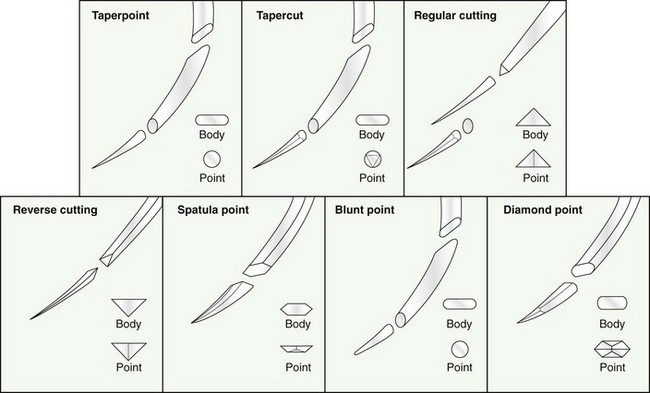
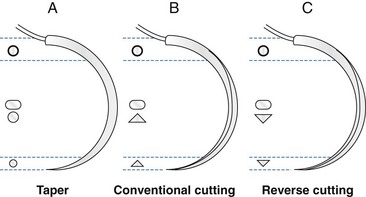
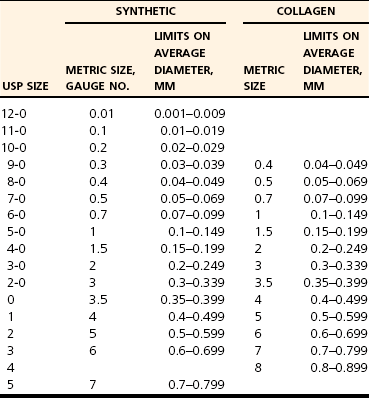
Chapter 17 Needles may be straight, curved, or a combination. The specific surgical task for which the needle is intended will dictate the ideal shape of the needle. Generally, straight needles are used closer to the surface of the body, almost exclusively in skin, because plentiful room is available for hand motion. For example, straight suture needles are useful for placement of tension-relieving sutures in the skin, where a moderate distance between the incision and the exit point of the suture is desirable. Curved needles are essential in small or deep surgical fields, where the size of the surgical field and working area is limited. Curved needles are further defined by the fraction of a circle ( Suture is available on either swaged (eyeless) needles or nonswaged (eyed) needles. Swaged needles are used most commonly because they are supplied with suture in single-use, sterile packs, are sharp because they are used only once, and cause less tissue trauma than nonswaged needles. Suture is attached with a channel swage or a laser-drilled swage. Channel-swaged needles have the suture crimped in a depression in the body of the needle, whereas laser-drilled needles have the suture crimped in a hole drilled in the body of the needle.81 Laser-drilled swages enable the surgeon to grip farther back on the needle and have less tissue drag because of a smoother needle-to-suture transition.2 Because of significant reduction in tissue trauma, swaged-on needles are essential for surgery in delicate settings such as ocular, vascular, urogenital, or intestinal procedures. Nonswaged suture needles are less commonly employed than swaged-on needles in small-animal veterinary surgery, but they can be useful for passing large suture in orthopaedic surgery or other specialized applications. Nonswaged needles are reusable and have either a closed eye or a ridged slit to hold suture during needle passage. Needles with a ridged slit are also referred to as French or split needles. With either type of nonswaged needle, suture should be loaded from the concave surface because the suture will be less prone to pulling out of the needle.15 When nonswaged needles are used, suture must double back on itself, causing the width of the suture attachment to be greater than the width of the needle; therefore, tissue trauma is increased during needle and suture passage compared with swaged-on needles. Additionally, routine needle use and repeated cleaning and sterilization will dull the needle and further increase tissue trauma. Because of these disadvantages, swaged-on needles are recommended for routine clinical use. Suture needle points, which are task specific (Figure 17-2), are broadly classified as blunt, taper, or cutting. Blunt needles can be used in friable tissue but in normal tissue have poor handling characteristics and are more difficult to use.156 Taper needles have a fine point that pierces and spreads tissue during needle passage. These needles are useful in delicate tissues such as gastrointestinal organs, fat, the urinary bladder, and muscle, especially in situations where the size of the needle hole must be kept as small as possible. Tapercut needles are a combination of a taper and a cutting needle. Tapercut needles have a reverse cutting point and an oval body, allowing greater ease of needle penetration with less risk of inadvertent tissue cutting or fraying during needle passage. In tough or fibrous tissue such as skin, periosteum, and fascia, cutting-type needles are selected to increase the ease of tissue penetration. Standard cutting needles are triangular and have the cutting surface on the concave surface of the needle. With standard cutting needles, a triangular defect is created during needle passage, in which the vertex created by the cutting edge of the needle is directed toward the incision (Figure 17-3). This needle hole configuration, along with the normal tendency of surgeons to put greater force toward the concave surface during needle passage, may result in a larger-than-ideal suture hole. This is problematic because the long axis of the needle hole will be perpendicular to the incision, which can result in increased risk of suture pull-through. To prevent such a complication, reverse-cutting needles have the cutting surface on the convex surface, resulting in a triangular needle hole with a flat edge parallel to the incision. Theoretically, this design should have less risk of inadvertent needle-hole elongation during needle passage and consequently less risk of suture pull-through. The function of suture is to hold tissue in apposition and carry all physiologic forces until wound healing progresses to the point that the tissue can sustain those forces.23 Several characteristics are necessary for consideration when a suture material is chosen. Sterility, uniform diameter and size, pliability for ease of handling, uniform tensile strength, a nonirritating composition, and freedom from impurities are essential qualities of suture material.81 The ideal balance of these characteristics will depend on its intended purpose. Suture is described and compared on the basis of its many mechanical and physical qualities. Suture size is standardized to U.S. Pharmacopeia (USP) standards (Table 17-1). Although a significant number of studies have evaluated suture performance, with the exception of USP data, experimental conditions (e.g., suture size, rate of force application, immersion environment) are frequently not standardized between scientific investigations. Thus, data from independent investigators regarding suture and knot strength, suture absorption, and general clinical performance are difficult to compare directly. However, surgeons should be aware of nomenclature used to describe experimental and clinical suture performance. The following terms have been used to describe the properties and characteristics of suture81: 1. Breaking strength: The stress value on the stress-strain curve at which suture acutely fails. 2. Capillarity: The degree to which absorbed fluid is transferred along a suture. Multifilament sutures tend to have greater capillarity compared with monofilament suture. 3. Creep: The tendency of a suture to slowly and permanently deform under constant stress. 4. Elasticity: The degree to which a suture will deform under stress or load and return to its original form when the load is removed. 5. Fluid absorption: The degree to which a suture will absorb fluid following immersion. 6. Knot pull-out strength: The load required to break a suture deformed by a knot. Deformation caused by knot placement generally results in a 10% to 40% loss of strength. 7. Knot strength: The force necessary to cause a knot to slip. 8. Memory: The tendency for a suture to return to its original shape after deformation. 9. Plasticity: The degree to which a suture will deform without breaking and will maintain its shape after removal of the deforming force. 10. Pliability: The ease of handling and the ability to change the shape of suture. 11. Stress relaxation: The ability of suture to reduce stress under constant strain. 12. Suture pull-out value: The weight required to pull a suture loop from tissue. The measured value relates to tissue strength (fat = 0.2 kg, muscle = 1.27 kg, skin = 1.82 kg, fascia = 3.77 kg). 13. Tensile strength: Similar to ultimate strength, breaking strength, or yield strength. It is a measure of a suture’s ability to resist deformation and breakage and the stress at which deformation (yield strength) or rupture (breaking or ultimate strength) occurs. The morphology of suture can be broadly classified as monofilament or multifilament. Monofilament suture consists of a single strand of material. Multifilament suture is a collection of multiple strands; the strands can be braided or twisted together. Monofilament suture tends to be less pliable and more susceptible to catastrophic damage from crushing or nicking.81 Monofilament suture has lower tissue drag, as the surface is much smoother compared with multifilament suture. Multifilament suture is composed of a braid or twist of smaller strands. Catgut, collagen, and cotton suture are available as a twisted multifilament, whereas most synthetic sutures are a braided multifilament.25 The braided configuration results in greater strength and pliability compared with a monofilament of the same material and size. However, multifilament suture has greater tissue drag or friction as it is pulled through tissue, which can result in bunching of tissue and increased tissue trauma around the path of the suture. Last, braided suture has greater capillarity and an increased tendency for bacterial colonization. Multifilament suture should be avoided in contaminated wounds or in applications where wicking of bacteria is undesirable. Newer sutures meant to carry high loads in orthopedic applications have a composite structure with multiple polymers. Some of these composite or “polyblend” sutures have a core of one polymer and a braided exterior composed of a different polymer. These sutures are extremely strong and resistant to failure, even when abraded or damaged.157 They are designed primarily for orthopedic applications and are discussed in detail later. A final variant in suture morphology is the presence of surface barbs. Self-anchoring, barbed sutures made from a monofilament suture base have been introduced in an attempt to eliminate bulky knots and intermittent, high-tension regions associated with loop sutures.104 In a clinical trial, barbed suture resulted in equivalent cosmesis, wound healing, and numbers of adverse events when compared with standard knotted suture.104 The security and utility of this suture morphology in veterinary surgical applications are unknown. Suture can be coated with a variety of compounds that are broadly characterized as water soluble or insoluble. For example, polyglactin 910 (Vicryl) suture is coated with a water-insoluble, 50 : 50 mixture of polyglactin 370 and calcium stearate, which serves to increase pliability, reduce tissue drag, and improve tying characteristics.128 Polyglycolic acid suture (Dexon II) is available with a coating of a water-soluble copolymer of glycolide and ε-caprolactone, which is designed to facilitate knot formation. A polyester suture (Ticron) is coated with a proprietary silicon compound for similar reasons. Although handling and tying characteristics are purported benefits of many coatings, water-insoluble coatings, unlike water-soluble coatings, will reduce knot security compared with similar uncoated suture.25,122,123 Antibiotic coatings are recently available on a number of sutures. Triclosan is a commonly used antibiotic suture coating and is an inhibitor of bacterial fatty acid synthesis with demonstrated antibacterial activity when applied to various sutures.54,64,101,102 Significant in vivo benefit of triclosan suture coating has been confirmed in animal models of operative contamination54,93,101,102,138 and in a nonrandomized, prospective clinical trial evaluating triclosan suture coatings for closure of laparotomy incisions in people.72 In the later study, polydioxanone suture with a triclosan coating (PDS Plus) reduced the percent of wound closures with postoperative wound infection from 11% (with uncoated polydioxanone suture [PDS II]) to 5%.72 Another study documented a significant reduction in postoperative pain when pediatric patients received triclosan-coated polyglactin 910 (Vicryl Plus) compared with standard polyglactin 910.49 In an attempt to improve wound healing, investigators have coated suture with various other compounds, including growth factors,38 bioactive glass,18,22,136 stem cells,160 matrix metalloproteinase inhibitors,111,112 and cyclooxygenase inhibitors.164 Although not specified by USP, absorbable suture is commonly defined as suture that loses most of its tensile strength within 60 to 90 days in living mammalian tissue (Table 17-2).25 USP defines absorbable suture simply as suture that is absorbed by living, mammalian tissue. The rate of suture absorption will vary, depending on material, coatings, size, biochemical environment, testing method, and species.23 The two major mechanisms of suture absorption are enzymatic and hydrolytic. Natural sutures, such as catgut and other collagen-based material, are absorbed through enzymatic actions of cellular proteases and collagenases.23 Specifically, acid phosphatase and leucine aminopeptidase play major enzymatic roles in the enzymatic absorption of catgut suture.126 Synthetic absorbable sutures are absorbed through hydrolysis by random main-chain scission of ester linkages.84 The absorption process can be a rapid, moderate, or slow process. The environment surrounding the suture is a critically important determinant of the rate of suture absorption. Thus, although general times for absorption are offered as guidelines for clinical use, considerable variation in absorption rate and residual strength is noted. Table • 17-2 General Characteristics of Common Absorbable Sutures +, Poor; ++, fair; +++, good; ++++, very good. Table adapted from Chu CC, von Franhofer JA, Greisler HP, editors: Wound closure biomaterials and devices, New York, 1997, CRC Press. As an example of the dramatic influence of environment on characteristics of suture absorption, exposure to urine can have a substantial influence on suture strength over time. In one study, when immersed in sterile urine, polydioxanone (PDS) lost all strength in 3 days, and polyglycolic acid (Dexon) lost 64% of its initial breaking strength after 10 days.41 In the same study, when urine was infected with Escherichia coli or Proteus bacteria, rates of absorption were dramatically increased. Polydioxanone and polyglycolic acid lost all strength after 1 day in Proteus-infected urine; however infected or sterile urine had little effect on catgut suture.41 Under similar conditions, other investigators noted that polydioxanone, poliglecaprone 25 (Monocryl), polyglyconate (Maxon), and glycomer 631 (Biosyn) disintegrated after 7 days in Proteus-infected dog urine.57 In the same study, immersion in sterile dog urine and E. coli–infected dog urine also resulted in a reduction, although less dramatic, in tensile strength.57 Another significant determinant of suture absorption qualities is environmental pH. Sutures with a glycolide component (poliglecaprone 25, polyglyconate, glycomer 631) will degrade more rapidly in an alkaline environment, whereas sutures with a dioxanone component (polydioxanone) will lose tensile strength rapidly in an acid environment.144 Similarly, in acidic conditions, nylon suture, which normally is considered a nonabsorbable suture, can lose 50% of its strength in 12 weeks, and polypropylene, another nonabsorbable suture, is resistant to reduction in tensile strength regardless of pH.144 Other investigators compared retention of tensile strength of polyglactin 910 (Vicryl) versus that of polyglycolic acid (Dexon) after immersion in neutral, acid, and alkaline solutions for up to 120 days. Polyglactin 910 had the least reduction in tensile strength in neutral solution but rapidly lost strength in both acid and alkaline solutions; polyglycolic acid lost more strength in alkaline urine, consistent with the results discussed previously.26 Other local variables have been identified to affect the rate of suture absorption. Exposure to bodily fluids such as pancreatic secretions, bile, gastric secretions, or blood has a variety of impacts on suture degradation, independent of pH.50 Immersion of suture in sterile or infected milk results in reduced suture strength.106 Temperature, as well as rate of application and amount of strain, also have an effect on the ability of suture to withstand tensile load over time.63,99 For example, polyglycolic acid is the most stable at around 37° C and is degraded by hydrolysis more rapidly at higher or lower temperatures; also, the slower and tighter a knot is tied, the greater is its ability to withstand tensile loads during hydrolysis.63 Prestraining suture material, as one might do clinically to reduce suture memory, will enhance suture degradation.99 Other factors such as irradiation, type of sterilization, presence of free radicals, type of environmental electrolytes, suture surface modifications, temperature, presence of bacteria, and presence of synovial fluid all have an impact on the rate of suture absorption.13,23,40,84 Catgut is a twisted suture material made from the small intestinal submucosa of sheep or the intestinal serosa of cattle and is available in plain or chromic forms. Compared with plain catgut, chromic catgut undergoes additional curing or tanning processes with chromium trioxide salts, which increases collagen cross-linkages, delays suture absorption, and reduces tissue inflammation.25 Catgut is relatively weak compared with other sutures. Because it is a natural substance, catgut is not uniform in diameter and has random thinner and weaker zones throughout its length. Additionally, tissue reaction to catgut suture is high and absorption is extremely variable depending on local conditions. Because catgut suture is absorbed by enzymatic processes, very rapid absorption occurs when it is used in gastric or intestinal surgery because of exposure to proteolytic enzymes. One study documented complete loss of strength of plain or chromic catgut within 12 or 24 hours, respectively, when used in the canine stomach.69 When used in bladder surgery, catgut can lose up to 90% to 100% of its original strength in 7 days.31,129 Regardless, in any environment, chromic or plain gut will be completely absorbed in 2 to 3 weeks. Polyglycolic acid is a braided homopolymer of glycolic acid and is available in a coated (Dexon II) or uncoated (Dexon S) form.16 Polyglycolic acid is absorbed completely in 60 to 90 days. During the first 14 days, minimal absorption is followed by more rapid hydrolysis and degradation. Polyglycolic acid has about 50% of its strength remaining at 2 to 3 weeks.25 When tested against polyglactin 910 (Vicryl), polyester (Mersilene), silk, nylon (Neurolon, Ethilon), and polypropylene (Prolene), polyglycolic acid had the highest breaking stress, was third to polypropylene and nylon in breaking strain, and was fourth in modulus of elasticity.27 Uncoated polyglycolic acid has been shown to have greater knot security compared with polyglactin 910.75,147 Polyglactin 910 is a multifilament braided copolymer of 90% glycolide and 10% L-lactide. Polyglactin 910 is coated with calcium stearate and polyglactic acid to improve its handling characteristics. Polyglactin 910 is available with an antibacterial triclosan coating (Vicryl Plus)54 or in an irradiated form with very rapid absorption characteristics (Vicryl Rapide).40 Polyglactin 910 is similar to polyglycolic acid in terms of mechanical and absorption characteristics, and it has about 50% of its original tensile strength at 2 to 3 weeks.25 Poliglecaprone 25 is a rapidly absorbed monofilament co-polymer of ε-caprolactone and glycolide.17 Poliglecaprone 25 has identical knot performance, as defined by loop holding capacity (N) and distance of knot sliding, compared with polyglactin 910, similar performance to polydioxanone, and lesser performance compared with polyglyconate (Maxon).148 Poliglecaprone 25 has high initial breaking strength, being superior to chronic gut, polyglactin 910, and polydioxanone (PDS II).17 Poliglecaprone 25 is also available with triclosan coating (Monocryl Plus). After implantation in rat subcutis, Poliglecaprone 25 will lose approximately 50% of its strength at 1 week and 70% to 80% at 2 weeks.17 Poliglecaprone 25 is completely absorbed in 90 to 120 days with mild tissue inflammation during the absorption period.17 Poliglecaprone 25 has identical knot performance to polyglactin 910, similar performance to polydioxanone, and lesser performance compared with polyglyconate.148 Polyglytone 6211 is a rapidly absorbed, monofilament polymer of glycolide, caprolactone, trimethylene carbonate, and lactide. Polyglytone 6211 has approximately twice the initial loop strength of chronic gut suture. After 3 weeks in rat subcutis, no measureable strength is seen in either polyglytone 6211 or chromic gut.115 Polyglytone 6211 has less tissue drag and knot security than chromic gut.115 Polydioxanone is a second-generation, uncoated, monofilament suture made from polydioxanone. It is available with a triclosan coating (PDS plus) or without (PDS II). The original form of polydioxanone (PDS) is no longer available. Absorption of polydioxanone is significantly prolonged compared with those sutures classified as rapidly absorbed synthetic sutures. Approximately 50% of its initial tensile strength remains at 5 to 6 weeks. Polydioxanone is similar to polyglyconate in terms of absorption and strength, but it has better handling and less memory. Generally, as compared with other sutures, polydioxanone has intermediate knot security (see Table 17-2). When compared specifically with polyglyconate suture regarding loop-holding capacity, polydioxanone has less knot security.147 Polyglyconate is a co-polymer of glycolic acid and trimethylene carbonate. Polyglyconate is a monofilament and a relatively slowly absorbed suture that is similar in many ways to polydioxanone. Polyglyconate has greater memory compared with polydioxanone, which detracts somewhat from its handling characteristics. Polyglyconate retains approximately 50% of its strength after 4 to 5 weeks. Compared with polydioxanone, polyglactin 910, coated polyglycolic acid, and poliglecaprone-25, polyglyconate had better knot performance when tested with the loop-holding method.146,148 Glycomer 631 is a monofilament absorbable composed of glycolide, dioxanone, and trimethylene carbonate. Glycomer 631 is relatively rapidly absorbed with approximately 50% loss of tensile strength at 2 to 3 weeks and is completely absorbed at 90 to 110 days.27 When compared with polyglactin 910, glycomer 631 was stronger and had greater first throw knot hold.120 Silk is a natural, braided, multifilament suture. Silk is produced by the silkworms Bombyx moorei and Anaphe pernyi and consists mostly of fibroin, a fiber protein, and sericin, a sticky substance that holds the fibers together.24 Although considered a nonabsorbable suture, the crystalline structure of silk is slowly degraded by hydration.24 Silk is completely deteriorated by 2 years, with significant loss of strength (56% of original tensile strength) in the first 12 weeks.58 Table • 17-3 General Characteristics of Common Nonabsorbable Suture +, Poor; ++, fair; +++, good; ++++, very good. *Break strength on straight pull, 0-3-0 suture. †Ref #66 (#2 FiberWire on straight loading). ‡Ref #59 (#4-0 suture on straight loading). Table adapted from Chu CC, von Franhofer JA, Greisler HP, et al, editors: Wound closure biomaterials and devices, New York, 1997, CRC Press. Silk is used frequently for ligation of large vessels because it has excellent handling characteristics and good knot security Additionally, the relatively high tissue reactivity of silk offers surgeons a strategy for chronic, progressive vascular occlusion. Silk is a foreign protein that incites a considerable tissue reaction, and it has been suggested that this foreign body reaction and the associated inflammation are responsible for progressive occlusion of partially ligated vessels over time.151 Specifically, this characteristic is thought to be advantageous when silk is used to partially ligate portosystemic shunts in animals.71,130,151 However, progressive venous attenuation was not verified in an experimental model of femoral vein attenuation.163 In the latter study, partial ligation of canine femoral veins with silk ligatures failed to result in significant progressive vessel attenuation after 6 weeks.163 The influence of suture location (femoral vein vs. peritoneal cavity) on inflammation and on silk’s propensity to progressively occlude vessels is unknown.130 Polypropylene is a monofilament polyolefin suture. Polypropylene has less knot strength when compared with polyglyconate and polydioxanone.146 Polypropylene is very strong and has the highest energy to break point (area under the displacement-load curve [N·mm]) when compared with silk, nylon, polyester, polyglycolic acid, and gut.77 Polypropylene has good handling characteristics and is commonly used in tendon, ligament, joint capsule, or fascia closures, which require prolonged suture strength. Polypropylene is very resistant to degradation because of a lack of hydrolyzable bonds.144 Nylon is a monofilament polyamide-based suture. Nylon is also a very strong suture. In one study, nylon was second to polypropylene in energy to break point (defined earlier) and had a very high maximal tensile load (measured in newtons).77 Despite classification as a nonabsorbable suture, nylon is susceptible to degradation.23,144 Degradation begins as a result of hydration soon after implantation; this may lead to disruption of hydrogen bonds and loss of tensile strength.23 In an acid environment, hydrolysis of amide linkages is catalyzed, and 50% loss of tensile strength can be expected in 12 weeks. Nylon fishing or leader line is frequently used in veterinary orthopaedic repair.131 Leader line is distinct from regular fishing line. Leader line, which is designed to connect fishing or fly line to the bait or tippet, is made to withstand high loads and is used primarily in saltwater fly fishing or fishing for larger fish with powerful jaws and sharp teeth. Leader line is an attractive material to many veterinary surgeons because of its ready availability and low cost; however, because this material was not designed as an implantable biomaterial, caution is advised in its use. Monofilament leader line will elongate under constant load, and its physical properties are significantly influenced by sterilization techniques.131,132 When the impact of steam or ethylene oxide sterilization on mechanical properties of nylon line was evaluated, steam sterilization resulted in a two- to four-fold increase in elongation.131 However, steam sterilization did not affect ultimate strength.131 Of the fishing materials and sterilization techniques tested, Mason leader line, sterilized with ethylene oxide, had the best strength and the least elongation.131 In another experiment evaluating mechanical properties and sterilization techniques of 36 kg test nylon fishing line, sterilization techniques again significantly influenced strength and cyclic elongation.132 “Ande” fishing line was the nylon material of choice when ethylene oxide was used for sterilization, and “Ande” and “Maxima” were the nylon materials of choice when steam sterilization was used.132
Suture Material, Tissue Staplers, Ligation Devices, and Closure Methods
Suture Needles
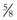 ,
, 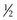 ,
, 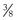 ,
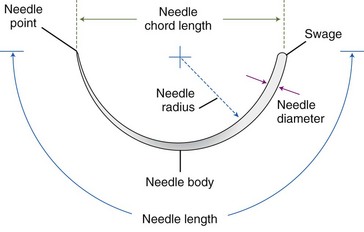
, 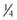 ) that they overlie, the cord length or bite width, the radius, the wire diameter, and the overall length or arc length (Figure 17-1). Combination needles with both curved and straight sections are called J-, ski-, or f-needles and have a straight body and a curved point. The j shape is preferred by some for intracorporeal endoscopic suturing, as the straighter body is optimal for gripping with endoscopic needle drivers.110
) that they overlie, the cord length or bite width, the radius, the wire diameter, and the overall length or arc length (Figure 17-1). Combination needles with both curved and straight sections are called J-, ski-, or f-needles and have a straight body and a curved point. The j shape is preferred by some for intracorporeal endoscopic suturing, as the straighter body is optimal for gripping with endoscopic needle drivers.110
Suture
General Suture Morphology
Suture Coating
Absorbable Suture
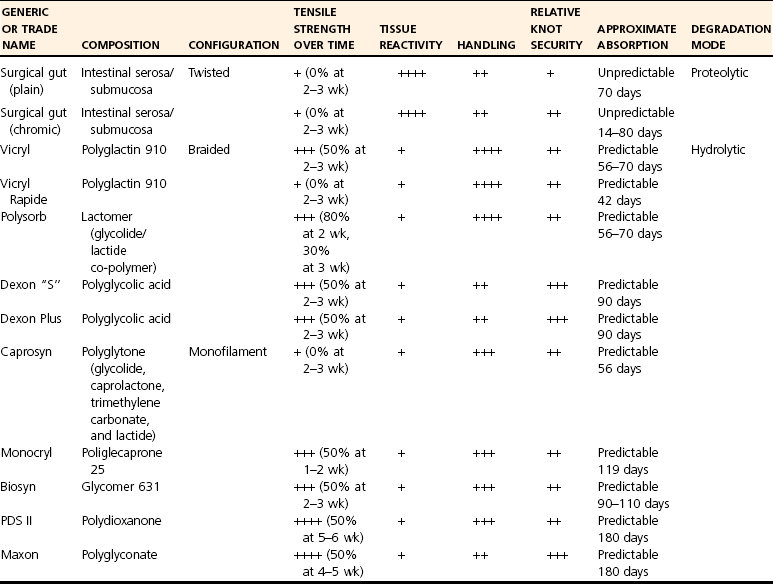
Common Types of Absorbable Suture
Rapidly Absorbed Synthetic Suture
Polyglactin 910: Vicryl
Poliglecaprone 25: Monocryl
Polyglytone 6211: Caprosyn
Common Slowly Absorbed Synthetic Sutures
Polyglyconate: Glycolic Acid Trimethylene Carbonate, Maxon
Glycomer 631: Biosyn
Common Nonabsorbable Sutures (Table 17-3)
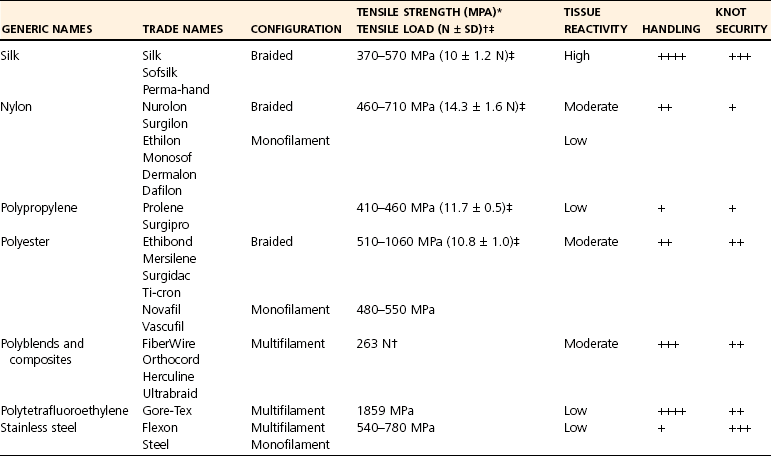
Polypropylene
Nylon
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Suture Material, Tissue Staplers, Ligation Devices, and Closure Methods
Only gold members can continue reading. Log In or Register to continue