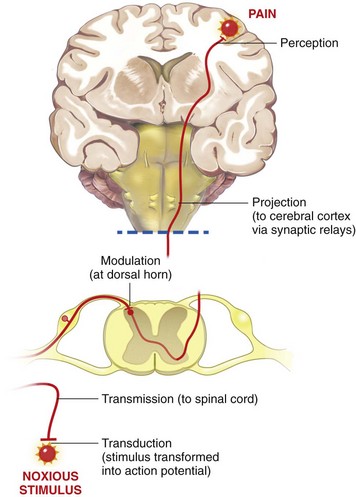
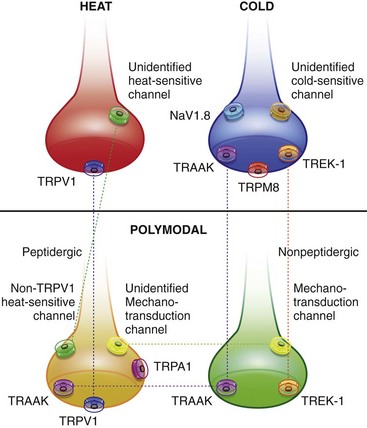
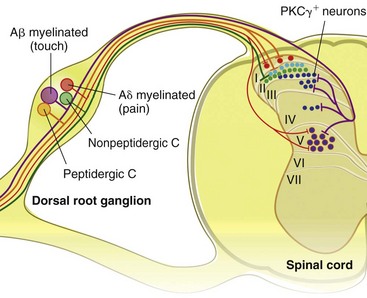
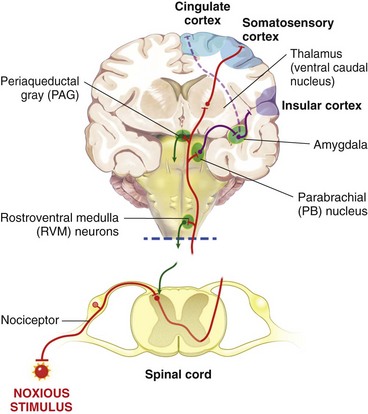
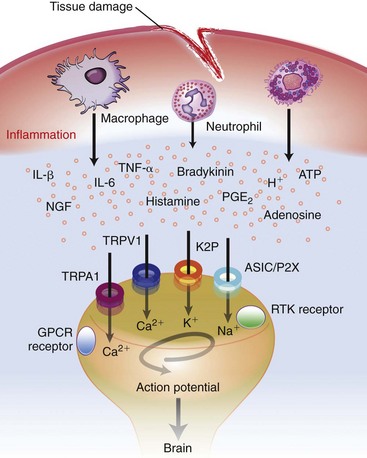
Chapter 22 Unlike other senses, such as vision, receptors for tissue damage do not return to their prestimulus state after activation but remain “switched on” for a period of time. It is now known that not only is the sensitivity of damage-detecting sensory nerve endings altered after injury, but their synaptic connections inside the central nervous system may be significantly modified and reorganized. The ability of the pain-sensing system to change in response to input is referred to as plasticity. The end result of these plastic changes is heightened activity and responsiveness of the system, making it easier for a signal to get through and be perceived as painful (hyperalgesia); stimuli that would not normally have been felt as painful (both from the site of surgery and from other areas) may be felt as painful when the animal regains consciousness (allodynia). Changes that occur in the peripheral and central nervous systems are known as peripheral and central sensitization. This sensitization can occur very quickly after surgery.46,47 The clinical implications of this sensitization include the following: (1) once “pain” is established, analgesic drugs, for a given dose, are much less effective (i.e., pain is more difficult to control), and (2) for a constant input over time, the “pain” felt by the animal increases. Sensory nerve fibers recognize and transform (transduction) environmental stimuli into electrical signals (action potentials) that are conveyed (transmission) to the dorsal horn of the spinal cord, where they are processed (modulation) and then relayed (projection) to the brainstem and brain, where the signal is integrated, recognized, and identified (perception) as pain (Figure 22-1). Nociception is the creation and transfer of signals after activation of nerve endings by a noxious stimulus. The term pain implies that noxious stimuli have been perceived at the thalamic or cortical level and interrupted as “pain.” Most of us believe (correctly) that the veterinary species we work with “feel” pain; however, some scientists still believe that these animals do not feel pain—that their reactions are simply a complex series of different reflex systems. Most nociceptors are relatively nonselective ion channels gated not by voltage but by the stimulus (temperature, chemicals, mechanical forces). Once activated, the channels open and the inward flux of Na+ and Ca2+ ions produces an inward current that depolarizes the membrane. The presence, specificity, and threshold of these transducers define the different classes of primary afferent fibers. Most fibers are considered polymodal, responding to multiple types of noxious stimuli, whereas some are unimodal and respond to only one form of stimulus. Almost all C-fibers are high-threshold and polymodal. Considerable progress has been made over the past decade in identifying the molecular structure and function of various nociceptor ion channels.5 Most C-fibers are polymodal, being both heat and mechanically sensitive (C-fiber nociceptors).63 Certain C-fiber nociceptors, called silent nociceptors, are heat responsive but mechanically insensitive, and are not normally active. These develop mechanical sensitivity only in the setting of injury and become more responsive when the so-called “inflammatory soup” alters their properties. Subsets of these C-fiber nociceptors afferents are also responsive to a variety of itch-producing substances. Approximately 10% of all C-fiber nerve endings respond to low-intensity stimuli, such as stroking, and may mediate pleasant touch. Nociceptors can also be distinguished according to their differential expression of channels that confer sensitivity to heat (transient receptor potential vanilloid-1 [TRPV1]), cold (transient receptor potential menthol-8 [TRPM8]), acid environment (acid-sensing ion channels), and a host of chemical irritants [TRPA1])5,41 (Figure 22-2). The human pain threshold to heat, which typically rests around 43° C, parallels the heat sensitivity of C and type II Aδ nociceptors described earlier. Capsaicin and related vanilloid compounds produce burning pain by depolarizing specific subsets of C and Aδ nociceptors through activation of the capsaicin (or vanilloid) receptor, transient receptor potential vanilloid-1—one of approximately 30 members of the greater transient receptor potential (TRP) ion channel family.13 Recent studies indicate that TRPV1 is not solely responsible for heat transduction but plays an important role. Current data indicate that both TRPV1-dependent and TRPV1-independent components of noxious heat sensitivity are mediated via TRPV1-expressing nociceptors. This is an important concept, because targeting TRPV1-expressing neurons (e.g., molecular neurosurgery) may have greater utility than selective targeting of TRPV1 receptors. Recent unpublished data from human patients support this. The somatosensory system detects diverse mechanical stimuli, ranging from a light brush of the skin to distention of viscera. High-threshold mechanoreceptors include C-fibers and slowly adapting Aδ mechanoreceptor fibers, both of which terminate as free nerve endings in the skin. Low-threshold mechanoreceptors include Aδ hair fibers that terminate on down hairs in the skin and detect light touch. Aβ fibers that innervate Merkel cells, Pacinian corpuscles, and hair follicles detect texture, vibration, and light pressure. Very little is known about the molecular basis of mechanosensation. Suggested candidate mechanotransducers include mec-4 and mec-10, members of the degenerin/epithelial Na+ channel (DEG/ENaC) families, possibly involving the MEC-2 orthologue, SLP3.86 Acid-sensing ion channels 1, 2, and 3 have been proposed as mechanotransduction channels; however although these channels appear to play a role in musculoskeletal and ischemic pain, their contribution to mechanosensation is controversial. TRPV2 and TRPV4 have also been proposed to be involved in mechanotransduction. In addition to the transduction elements, other molecules are considered to play a role in transduction by tuning or altering sensitivity of the transduction elements. These are far from being well characterized but include voltage-gated sodium channels and voltage-gated potassium channels (KCNK2 [TREK-1] and KCNK4 [TRAAK]1,61 and KCNK18).7 If the depolarizing current initiated during transduction is of sufficient magnitude, voltage-gated ion channels are activated, further depolarizing the membrane and causing a burst of action potentials. Voltage-gated Na+ and K+ channels are critical to the generation of action potentials. Several isoforms of Na+ channels have been recognized for their specific role in nociception. The tetrodotoxin (TTX)-sensitive Na+ channel, Nav1.7, and the TTX-resistant Na+ channels, Nav1.8 and Nav1.9,5,14 have attracted the most attention. The Nav1.7 has received attention because of the finding that altered activity in this channel leads to a variety of human pain disorders,24,33,67 and the finding that Nav1.7 is upregulated in a variety of rodent inflammatory pain models.60 The Nav1.8 is also highly expressed by most C-fiber nociceptors and is not inactivated at low temperatures, making it the predominant action potential generator under cold conditions. Much of the analgesic effect of cold is considered to be due to inactivation of sodium channels. These voltage-gated sodium channels are the targets of local anesthetics and are the focus of intense development of specific channel blockers (such as Nav1.7 blockers). Additionally, many drugs have some sodium channel blocking activity. For example, the analgesic effects of serotonin and norepinephrine reuptake inhibitors may result in part from their ability to block sodium channels.25 Sensory nerve fibers enter the spinal cord primarily through the dorsal nerve root, but some fibers enter through the ventral nerve root as well. Nociceptive fibers enter the dorsal horn of the gray matter, where they are somatotopically oriented into laminae or layers and synapse with dorsal horn neurons (Figure 22-3). Many of the fibers bifurcate, sending branches that ascend and descend several spinal cord segments before entering and synapsing in the dorsal horn. Sensory nerve fibers can synapse directly with projection neurons, which relay information to the brain, or with local excitatory and inhibitory interneurons, which regulate and modify sensory information before it is relayed to projection neurons. Sensory input from the head region enters the central nervous system via the trigeminal nerve. Noxious information is relayed to the nucleus caudalis, which is part of the trigeminal sensory complex, and, in turn, these neurons project to the ventroposterior thalamus and reticular formation. Primary afferent nerve fibers project to the dorsal horn of the spinal cord, which is organized into anatomically and functionally distinct laminae (see Figure 22-3). C-fibers terminate in laminae I and II; Aδ terminate in lamina I and the deeper dorsal horn, lamina V; Aβ afferents (which under normal conditions respond to light touch) terminate in laminae III, IV, and V. Even within this organization, further suborganization is evident. Most peptidergic C-fibers terminate within lamina I and the most dorsal part of lamina II. In contrast, the nonpeptidergic C-fiber afferents, including the Mrg-expressing subset, terminate in the midregion of lamina II. Lamina II is also referred to as the substantia gelatinosa. These afferent terminals synapse with dorsal horn neurons. Most transmission between primary afferents and dorsal horn neurons occurs through postsynaptically located ionotropic α-amino-3-hydroxyl-5-methyl-4-isoxazole propionic acid (AMPA) receptors with a small N-methyl-D-aspartate (NMDA) component. Consistent with this organization, electrophysiologic studies have evaluated the responses of spinal cord neurons within these laminae: spinal cord neurons within lamina I are generally responsive to noxious stimulation (via Aδ and C-fibers) and are termed noxious-specific neurons; neurons in laminae III and IV are primarily responsive to innocuous stimulation (via Aβ); and neurons in lamina V receive convergent nonnoxious and noxious input via direct (monosynaptic) Aδ and Aβ inputs and indirect (polysynaptic) C-fiber inputs, and are termed wide dynamic range neurons, in that they respond to a broad range of stimulus intensities. Wide dynamic range neurons respond in a graded manner over large receptive fields, often receive convergent deep and visceral input, and likely contribute to the phenomenon of referred pain, whereby noxious signals from a visceral tissue are referred to a somatic structure. Noxious-specific neurons have smaller receptive fields compared with wide dynamic range neurons and function in stimulus localization and discrimination. Wide dynamic range and noxious-specific neurons project to the reticular formation and thalamus of the brainstem by way of multiple parallel pathways called tracts, including the spinothalamic and spinoreticulothalamic tracts, the spinocervicothalamic tract, and the spinomesencephalic tract. Considerable species variation in the relative importance of these tracts with regard to nociception is likely. Data from human beings suggest that projections to the thalamus are concerned with the sensory-discriminative aspects of the pain experience (i.e., where it is, how intense it is), whereas projections to the brainstem may be more important with regard to poorly localized pain. Spinal cord projections to the parabrachial region of the dorsolateral pons, which provide a very rapid connection with the amygdala, are likely associated with processing of aversive properties of the pain experience. Noxious information reaches cortical structures via the brainstem and thalamic areas (Figure 22-4). The sensory-discriminative aspects of pain are produced in the lateral thalamocortical system, which consists of relay nuclei in the lateral thalamus and the primary and secondary somatosensory cortices. Motivational and affective aspects of the pain experience arise in the medial thalamocortical system, which includes relay nuclei in the medial thalamus that send projections to limbic structures, such as the anterior cingulate gyrus and prefrontal cortex, but that also includes spinal projections to the hypothalamus and amygdala (see Figure 22-4). Human imaging studies also demonstrate activation of prefrontal cortical areas and the basal ganglia and cerebellum during noxious stimulation. It is important to recognize that powerful descending pathways are able to modulate the pain response. Descending modulation is controlled from three main sources: the periaqueductal gray matter of the midbrain; the rostroventral medulla and pons of the brainstem; and the thalamocortical structures (see Figure 22-4).52 Descending neurons from the neocortex and hypothalamus activate enkephalinergic interneurons in the periaqueductal gray matter. The caudate nucleus and the amygdala, two other forebrain structures, have also been implicated in antinociception. Projections from the periaqueductal gray matter terminate in various brainstem structures in the rostroventral medulla, including the nucleus raphe magnus, the reticularis magnocellularis, and the nucleus paragigantocellularis. Descending serotonergic fibers originate in the nucleus raphe magnus; serotonergic, noradrenergic, enkephalinergic and joint enkephalinergic, and serotonergic fibers originate in the nucleus paragigantocellularis; and noradrenergic axons originate from cell groups lateral to the nucleus paragigantocellularis. These fibers descend in the dorsal lateral funiculus and synapse onto thalamic projection neurons in lamina I, and local opioid-containing inhibitory interneurons in lamina II (the substantia gelatinosa). These descending pathways are often activated by stressful events (pain, fear, hypoxia), and opioid-like analgesic effects are produced. Within the rostroventral medulla are populations of cells referred to as on and off cells.53 Off cells hyperpolarize in response to spinothalamic tract input and reduce the transmission of nociceptive signals through the brainstem. On cells are excited by nociceptive input from the spinothalamic tract and facilitate nociceptive transmission to areas involved in arousal and aversive reactions to pain. Activity of these neurons can also affect local spinal cord modulation of nociceptive processing. Control of these rostroventral medulla neurons is being investigated. 1. Gate control theory55: inhibitory interneurons normally inhibit output of projection neurons. Input from large myelinated Aβ fibers encoding non-noxious information can increase inhibitory interneuron activity, and limit the amount of noxious sensory information going to the brain. 2. Endogenous opioids: endogenous opioids inhibit the release of local excitatory neurotransmitters, including glutamate and substance P. 3. Other local modulators of excitatory and inhibitory spinal cord transmission: these substances are under local control or control via projections from higher centers. A surgical procedure, trauma, or disease results in direct stimulation of afferent nociceptors (Aδ and C-polymodal) or stimulation via the “inflammatory soup” that is produced. Neurotransmitters (glutamate), peptides (substance P, calcitonin gene-related peptide, bradykinin), eicosanoids and other lipids (prostaglandins, thromboxanes, leukotrienes, endocannabinoids), neurotrophins (nerve growth factor), cytokines, chemokines, proteases, and protons are all components of the so-called inflammatory soup. These substances are released from activated nociceptors, damaged tissue cells, or non-neuronal cells that reside in the local area or are recruited in platelets, neutrophils, lymphocytes, macrophages, and mast cells.49,54 Direct tissue damage results in local release and spread of adenosine triphosphate (ATP), ions (H+, K+), prostaglandins, bradykinin, and nerve growth factor. Lymphocytes, neutrophils, and macrophages release cytokines (interleukin [IL]-1, IL-6, tumor necrosis factor [TNF]-α). Mast cell degranulation increases the local concentration of 5-HT and histamine. These chemicals will directly activate or will sensitize nociceptors to different types of stimuli, such as thermal, mechanical, and chemical.49,54 The processes are complex and interrelated (Figure 22-5).
Surgical Pain
Pathophysiology, Assessment, and Treatment Strategies
Transduction (Peripheral Nociceptors)
Heat Transduction
Mechanical Transduction
Local Modulation of Transduction
Transmission and Projection
Dorsal Horn Neurons and Ascending Spinal Tracts
Thalamocortical System
Supraspinal Modulation of Sensory Input
Local Modulation of Sensory Input at the Spinal Cord
Plasticity of Nociception and Pain
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgical Pain: Pathophysiology, Assessment, and Treatment Strategies
Only gold members can continue reading. Log In or Register to continue