Chapter 14 Surgery of the Calf Gastrointestinal System
14.1 Abomasal Disease
A few noteworthy physiological differences exist between calves and adult cattle (see Section 10.4). The parietal cells in the fundic region responsible for secretion of HCl are essentially inactive in the neonate, and abomasal fluid has a pH near 6.0. The neutral pH, lack of proteolytic enzymes, and diversion of milk through the reticular groove are all essential to allow colostrum to pass rapidly through the abomasum into the duodenum, where immunoglobulins can be absorbed intact. However, parietal cells are active, and the pH of fundic secretions reaches adult pH levels by 36 hours after birth. Suckling also stimulates rennin and pepsin secretion from chief cells in the fundus of the abomasum, which is essential for clot formation in the diverted milk. The volume of milk consumed and the nature of duodenal chyme regulate abomasal emptying in the milk-fed calf. Somatostatin, secretin and cholecystokinin are hormones that inhibit abomasal emptying in calves. Introduction of hyper- or hypotonic solutions can also depress abomasal motility in milk-fed calves in which normal abomasal contents are essentially isotonic.
Abomasal Displacement
The clinical presentation of abomasal disease seen in calves varies. The abomasum can displace without volvulus to the left or right (LDA or RDA) of its normal position by swinging, folding, or stretching the lesser omentum and attached structures. Figure 14.1-1 shows that the abomasum and its attachments can stretch to the point at which the abomasum can be exteriorized almost completely through the left paralumbar fossa. In addition, dilation without apparent displacement is also described in calves. Abomasal volvulus (RVA) is seen in calves and seems to progress quickly if untreated. Finally, abomasal perforations secondary to abomasal ulcers are prevalent in calves.
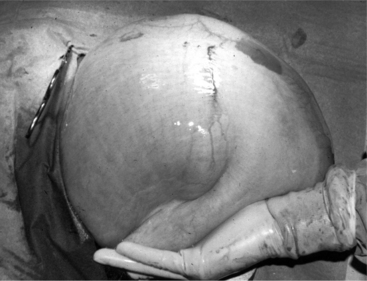
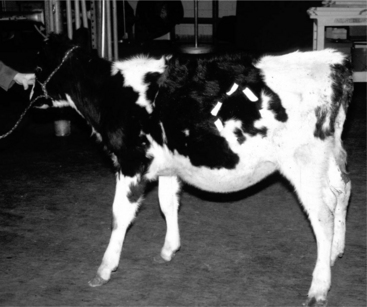
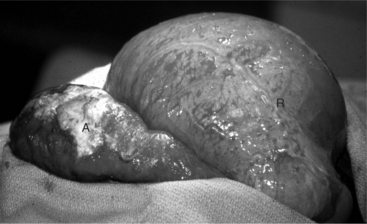
Figure 14.1-1 Standing left paralumbar fossa celiotomy in a 6 month old calf with LDA. The abomasum is exteriorized before decompression.
(Courtesy of Dr. Norm G. Ducharme; Cornell University.)
Left displacements are recognized occasionally in beef and dairy calves, most commonly between 3 weeks and 4 months of age, but the dietary predisposing factors are different; high amounts of starch in milk replacer and abomasal ulcers have been reported as factors in the pathogenesis of LDAs. The prevalence of abomasal ulcers in veal calves has been estimated at 39% of LDA cases.
DIAGNOSIS
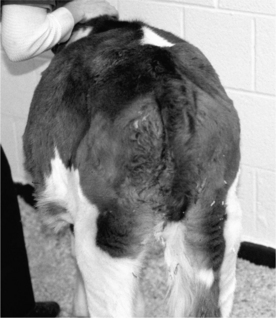
Clinical signs of left displacement are less well-described in calves but are more variable than in adults. They include nonspecific signs such as reduced appetite, poor weight gain, recurrent tympany, and variable fecal consistency. Unlike adult cattle, the distended abomasum typically fills the left flank in affected calves and can cause distinct asymmetry when viewed from behind or above (Figure 14.1-2). The ping detectable by simultaneous auscultation and percussion may be less high-pitched than in adult cattle, thus making it easily confused with ruminal distention. This may be why the diagnosis is sometimes delayed in calves. Sometimes the area of percussion is lower than one would expect adding to the diagnostic difficulty (Figure 14.1-3). Calves may also have concurrent diseases typical of their age group including pneumonia and diarrhea. As noted above, calves do not consistently demonstrate the hypochloremic metabolic alkalosis shown by adult ruminants with LDA, and laboratory evaluation is highly recommended if possible. Ultrasound evaluation can be very useful as well.
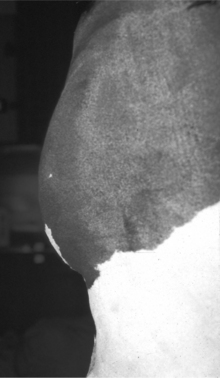
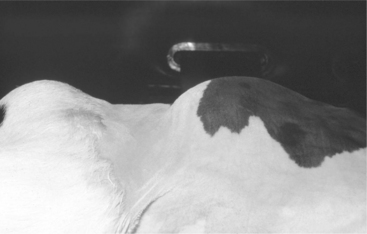
Figure 14.1-2 3-month-old calf with large LDA viewed from above. Note the prominent left-sided abdominal distention.
(Courtesy of Dr. Thomas Divers; Cornell University.)
TREATMENT
Some aspects of medical therapy are valuable adjuncts to surgical correction of gastrointestinal disturbances in calves. The potential for rapid dehydration dictates that systemic therapy begin before laboratory results can be obtained, even in calves with mild dehydration. Isotonic saline or lactated Ringer’s solution with supplemental dextrose is generally a safe choice until laboratory analysis is available. Oral supplementation should not be used as the primary method of rehydration. Oral and systemic intestinal stimulants have not been well evaluated in calves with abomasal displacement and are not recommended. Initiation of oral or systemic therapy for abomasal ulcers may be appropriate because of the association of displacement with ulcers in calves (see Chapter 5 for more details on fluid therapy). Appropriate medical therapy for other concurrent calf diseases such as pneumonia should be initiated.
MEDICAL MANAGEMENT
Contrary to the relatively poor outcome in adult cattle, rolling may be more effective in managing abomasal dilation as well as right and left displacement in calves. Successful management in 19 out of 21 calves without distinction between left and right displacements was reported in one study after rolling (see Section 10.4 for a description of the procedure). However, the editors feel that this should be a temporary measure to be used only in sick calves that cannot tolerate immediate surgery.
SURGICAL TREATMENT
Standing procedures are not generally recommended in small calves because of their tendency to lie down mid-procedure and the difficulty for the surgeon. However, larger calves may tolerate standing surgery. An omentopexy can be performed from a right flank approach in a calf in left lateral recumbency or standing; however, the omentum is fragile in calves and other approaches that directly stabilize the abomasum are preferable. A left paralumbar fossa abomasopexy is another surgical option (see Figure 14.1-1). This can also be done in the standing or recumbent animal. If the clinician is unsure whether the left-sided viscus is rumen or abomasum this is an attractive surgical option.
Abomasal Volvulus
The presence of a tympanic area (ping) centered over the 10th to 13th ribs and on a line from elbow to tuber coxae is the primary diagnostic sign of an RDA or RVA. This ping must be differentiated from other sources of right-sided pings, which include cecal dilation/torsion, gas accumulation in the duodenum, spiral colon, ascending colon or small intestine, or right flank abscess. An abomasal ping generally can be differentiated from pings associated with other structures by combining information about ping location, size, and pitch. The cecum, colon, and small intestines are limited in their mobility by their mesenteric attachments to the dorsal body wall, whereas the abomasum and ascending duodenum are limited by more cranial attachments at the duodenal sigmoid flexure and the reticular connection to the diaphragm. Cecal, colonic, and small intestinal pings will generally be centered on a point in the paralumbar fossa, more caudal than that for the abomasum or duodenum. The cecum is the only structure that has the potential to dilate to the maximum size possible for the abomasum and is the primary differential for single-pitched pings greater than 10 cm in diameter. In addition, to differentiate the ping’s center by location, the outline of the cecum can usually be seen through a right paralumbar fossa with the calf in lateral recumbency (see Figure 14.2.3-1). Small intestinal pings are typically a collection of small diameter pings of varying pitch. Spiral colon pings also tend to involve multiple areas that vary in pitch. Ultrasound evaluation may be very useful to differentiate the right sided viscus. The presence of tachycardia (>130/min) and colic have been reported to be more consistent with RVA in calves; however, controlled studies are lacking.
A right paracostal approach can be used to perform an abomasopexy (preferred procedure) in calves positioned in left lateral recumbency. A rolling procedure with percutaneous decompression (as described for LDAs previously) has been reported to be effective in calves with right-sided pings; however, this is risky should the calf have an RVA or involvement of another right-sided viscus. The veterinarian should be prepared to move to an open approach within 2 to 3 hours if clinical signs do not improve. A modified right flank or right paracostal omentopexy (Figure 14.1-4) can be used to correct abomasal displacement or volvulus in calves in left lateral recumbency. However, the limited holding power of the omentum in calves makes this a less desirable approach than the right paramedian or right paracostal abomasopexy described earlier.
Luminal Obstruction
Intraluminal obstructions are more common in calves than in adult cattle. Grooming behavior in group-housed calves can result in multiple cases of trichobezoars (hairballs) (Figure 14.1-5). As for LDAs in calves, the specific acid-base and electrolyte changes that result from outflow obstruction are more variable.
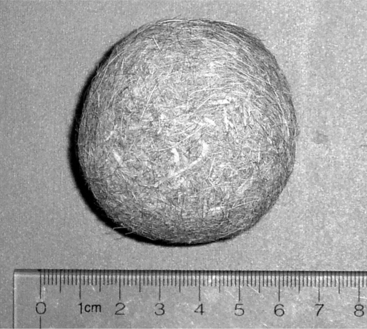
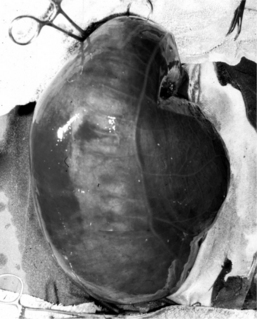
Figure 14.1-5 Trichobezoar removed from a calf’s abomasum.
(Courtesy of Dr. Brad L Njaa; Cornell University.)
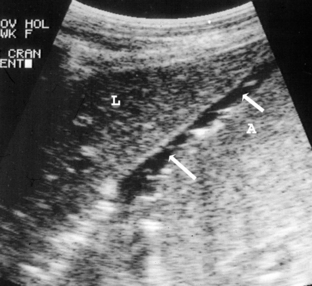
Ileus can lead to abomasal dilatation. Excessive milk consumption and changes in nutrition from milk to solid feed have also been suggested as a cause of abomasal distention without displacement in calves. Abomasitis is recognized as an inflammatory condition in young calves presumably as a result of infection with Clostridium perfringins, Sarcina sp., or Salmonella typhimurium. Affected calves present with signs of toxemia, including dehydration, abdominal distention, tachycardia, and a fluid filled abomasum in normal position on ultrasound examination (Figure 14.1-6).
Abomasal Ulceration
In one study of calves with clinically apparent abomasal ulceration, 17 of 118 animals had bleeding ulcers. Perforating ulcers (Types 3 and 4) appeared to be most common in veal calves, beef calves, and yearling feedlot cattle (Figure 14.1-7). The incidence in beef and veal calves was highest at 4 to 8 weeks of age, with most cases occurring by 12 weeks of age. Of clinically apparent ulcers in a 3-year study of calves, 81 of 118 were perforating. The occurrence of perforating ulcers in beef calves is highest in the spring, reflecting the age of greatest risk. The incidence of fatal ulcers (Types 2, 3, and 4 combined) was highest in the winter in yearling feedlot cattle but occurred throughout the year. Ninety-three percent of 209 fatal ulcers in beef calves were perforating.
Carlson SA, Stoffregen WC, Bolin SR. Abomasitis associated with multiple antibiotic resistant Samonella enterica serotype Typhimurium phagetype DT 104. Vet Microbiol. 2002;85:233-240.
Dirksen G. Ulceration, dilatation and incarceration of the abomasum in calves: clinical investigations and experiences. Bov Pract. 1994;28:127-135.
Frazee LS. Torsion of the abomasum in a 1-month old calf. Can Vet J. 1984;25:293-295.
Grymer J, Johnson R. Two cases of bovine omental bursitis. J Am Vet Med Assoc. 1982;181:714-715.
Jelinski MD, Janzen ED, Hoar B, et al. A field investigation of fatal abomasal ulcers in western Canadian bred calves. Agri-Practice. 1995;16:16-18.
Jelinski MD, Ribble CS, Campbell, Janzen ED. Investigating the relationship between abomasal hairballs and perforating abomasal ulcers in unweaned beef calves. Can Vet J. 1996;37:23-26.
Kümper H. A new treatment for abomasal bloat in calves. Bov Pract. 1995;29:80-82.
Roeder BL, Chengappa MM, Nagaraja TG, Avery TB, Kennedey GA. Experimental induction of abdominal tympany, abomasitis, and abomasal ulceration by intraruminal inoculation of Clostridium perfringens type A in neonatal calves. Am J Vet Res. 1988;49:201-207.
Roeder BL, Chengappa MM, Nagaraja TG, Avery TB, Kennedey GA. Isolation of Clostridium perfringens from neonatal calves with ruminal and abomasal tympany, abomasitis, and abomasal ulceration. Am J Vet Res. 1987;190:1550-1555.
Tulleners EP, Hamilton GF. Surgical resection of perforated abomasal ulcers in calves. Can Vet J. 1980;21:262-264.
Welchman D de B, Baust GN. A survey of abomasal ulceration in veal calves. Vet Rec. 1987;121:586-590.
14.2 Ruminal Distension in Calves
Diet inadequate in roughage can prevent normal growth of ruminal flora and is the most common cause of indigestion in calves. Undigested roughage accumulates and ruminal distention develops. It has been suggested that exclusively milk (or milk replacer) diets can cause hyperkeratosis of the rumen and recurrent ruminal distention. A similar syndrome is called ruminal drinkers where a calf’s esophageal groove partially or completely fails to close, so ingested milk is diverted to the rumen instead of the abomasum. This leads to fermentation and ruminal distension (Figure 14.2-1). Why this syndrome develops is unclear, but the following factors must be present for the esophageal groove to close normally. The fluid drunk by a calf must contact the pharyngeal receptors, be consumed voluntarily, and have no unpleasant taste or odor; and the general status of the calf should not be disturbed. Altering the method of intake or weaning the calf can be curative.
Another source of ruminal distention in calves is vagus nerve impairment due to pharyngeal disorders or chronic severe bronchopneumonia. The vagus nerve can ap-parently become inflamed or compressed by enlarged lymph nodes or severe pulmonary parenchymal damage. Because this nerve provides innervation to the forestomach compartments, an abomasum outflow obstruction develops with distention of the dorsal and ventral sacs of the rumen (Figure 14.2-2).

Figure 14.2-2 “Papple-shaped” abdomen in a calf with chronic bronchopneumonia and presumed vagal nerve damage.
(Courtesy of Dr. Thomas Divers; Cornell University.)
Clinical Findings
Pharyngeal trauma can be diagnosed by physical examination findings that include swelling, dysphagia, and excessive salivation (see Section 10.1). Thoracic lesions can be identified based on physical examination and imaging studies. For example, signs of bronchopneumonia, such as coughing, tachypnea, abnormal auscultation (wheezes, squeaks, crackles or decreased lung sounds), and dullness on thoracic percussion, help identify a primary respiratory problem requiring treatment. Imaging of the head or thoracic cavity by ultrasonography or radiography could confirm a diagnosis of trauma or bronchopneumonia.
Surgical Technique
The purpose of surgery is to allow decompression of the rumen, thus giving the primary condition an opportunity to resolve. The fistula should not be too ventral, or ingesta will tend to occlude the fistula, thus allowing ruminal distension to recur. The fistula is placed in the dorsal aspect of the left paralumbar fossa. However, the fistula should not be too dorsal if the rumen is empty (i.e., an animal with tetanus), or it will cause excessive tension on the suture line. The appropriate area on the left flank is identified, desensitized with a local block, and prepared for surgery. A 6-cm vertical skin incision is made. The external abdominal oblique and transversus muscles are incised sharply. The peritoneum is tented and incised.
Several surgical options are available, including the three-layer technique the authors use. We recommend the transversus abdominis (which is mostly fascia) and peritoneum be sutured to the dermis on each side of the incision for the first layer. This protects the muscle layers and is usually done with a size 0 absorbable suture on a cutting needle. For the second layer, the rumen wall is sutured circumferentially to the dermis to provide a “seal” between the rumen and the skin. For this layer, a cutting needle is required; and the suture line is “broken” several times to avoid a pursestring effect. For the third layer, the rumen is incised, and the wall is sutured to the skin in a simple interrupted pattern with size 0 nonabsorbable sutures. The ends are left long to facilitate removal. To keep the fistula patent, an appropriate-sized syringe case (with four holes in its collar) is fitted into the incision site. The syringe case is secured to the skin with umbilical tape placed through the preplaced holes in the collar to four separate “loops of sutures,” placed in the skin at four corners approximately 5 cm from the fistula (Figure 14.2-3).
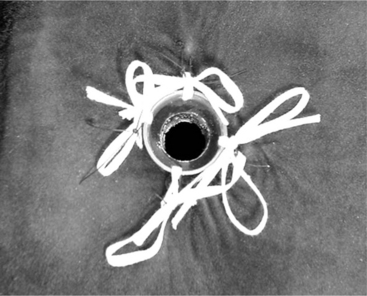
Figure 14.2-3 Completed rumen fistula in a calf.
(Courtesy of Dr. Brett Woodie; Cornell University.)
The syringe case is capped when the primary cause of the ruminal distension appears to have resolved. If the ruminal distension does not recur after a few weeks, the syringe case is removed. In some calves, the fistula will close over the ensuing weeks. In other calves, the fistula will need to be resected en bloc and the rumen and body wall closed separately as described in Section 10.3 for a rumenotomy closure.
Dirr L, Dirksen G. Dysfunction of the esophageal groove (“ruminal drinking”) as a complication of neonatal diarrhea in the calf. Tierarztl Prax. 1989;17:353-358.
Ducharme NG. Surgical considerations in the treatment of traumatic reticuloperitonitis. Compend Contin Educ Pract Vet. 1983;5:S213-S224.
Ducharme NG. Surgery of the bovine forestomach compartments. Vet Clin North Am (Food Anim Pract). 1990;6:371-397.
Habel RE. A study of the innervation of the ruminant stomach;. Cornell Vet. 1956;46:555-633.
Neal PA, Edwards GB. “Vagus indigestion” in cattle. Vet Rec. 1968;82:396-402.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree