Chapter 12 Surgery of the Bovine Reproductive System and Urinary Tract
Applied Anatomy and Examination of the Reproductive Tract
The testicular artery courses toward the deep inguinal ring of the inguinal canal, where it joins the spermatic cord. The latter structure consists of the testicular artery and vein, the lymphatics, nerves, and the ductus deferens. Together they pass through the inguinal canal. The testicular artery, once external to the abdomen, becomes extremely tortuous. In this area it is in close apposition to the dilated and contorted veins. This complex is known as the pampiniform plexus. It serves multiple purposes. The multiple contortions of the artery help blunt the pulsatility of arterial blood flow. (The fibrous outer surface of the testis, the tunica albuginea, is unyielding and extremely sensitive to distension, which means that arterial pulsatility within the testis would be most uncomfortable.) In addition, arterial blood is cooled by exposure to the effluent venous blood, and countercurrent exchange of steroids, especially testosterone, which is produced in the testis and is necessary in high concentrations locally for optimal testicular function. Testicular arterial blood is therefore cooler, is fortified with testicular steroids, and flows with diminished pulsatility.
The accessory sex glands are palpable per rectum in bulls. The bulbourethral glands can be felt as smooth structures that protrude from under the cranial edge of the bulbospongiosus muscles. The pelvic urethra, surrounded by the urethralis muscle, is prominent in the midline of the pelvic floor. If any doubt exists, the urethralis muscle can be identified by massaging it per rectum; it will respond by contracting rhythmically. As the urethralis muscle is traced forward, a transverse ridge can be felt at its cranial-most end. This is the body of the prostate. Converging on this point, the fairly soft, pliable ampullae can be felt. The ampullae move as a pair within the genital fold and are separated by less than half an inch. They may be displaced to one or the other side by a full bladder. Also converging toward the body of the prostate are the vesicular glands. These lobulated structures project craniolaterally. In young animals they are smaller and softer than in mature bulls.
Blocking the pudendal nerve achieves relaxation and desensitization of the penis and allows surgical procedures on the penis of the standing, restrained bull, provided the animal’s temperament permits. The pudendal nerve can be palpated per rectum. The internal pudendal artery serves as a landmark. It is readily palpable (per rectum) as it courses along the medial surface of the sacrosciatic ligament just dorsal to the ischiatic arch. If the artery is followed caudally, the lesser ischiatic foramen can be identified at the point where the artery divides. The pudendal nerve can be felt about 1 cm dorsal to the artery at the cranial edge of the lesser ischiatic foramen. It is convenient to block the nerve at the foramen and remember that it receives a branch from the caudal cutaneous femoral nerve at that point. The caudal cutaneous femoral nerve runs laterally to the sacrosciatic ligament. To administer a pudendal nerve block, the rectum is evacuated and the skin of the ischiorectal fossa disinfected. A bleb of local anesthetic agent (e.g., 2% lidocaine) is injected under the skin at the notch between the tail fold and the caudal extent of the sacrosciatic ligament, and a 15-cm, 18-gauge needle is inserted. The needle is guided toward the lesser ischiatic foramen with a hand in the rectum and the left hand is used to guide the needle for injection of the right nerve, and vice versa. The author prefers to inject about 10 to 12 ml at the site of the foramen, near the palpable pudendal nerve, and then divert the needle through the foramen to inject about 5 ml on the lateral side of the ligament, thus ensuring that the caudal cutaneous femoral branch is blocked. As the needle is withdrawn, the remaining 3 to 5 ml is injected slowly, in the hope of blocking the caudal rectal nerve. This nerve, although large, is not readily palpable per rectum because it moves with the rectal wall. It provides some innervation to the proximal fibers of the retractor penis muscle. Both pudendal nerves need to be blocked to produce relaxation of the retractor penis muscles and analgesia of the penis. The penis may prolapse out of the prepuce spontaneously after this procedure, but more commonly it is retained within the prepuce by passive mechanisms (loose connective and elastic tissue surrounding the prepuce), and it needs to be extended manually.
The flaccid and insensitive penis should be extended fully, by grasping the free end of the penis with a gauze swab (see Figure 12.1.5-5). Forceps may be used to secure the penis, with care taken not to penetrate the tunica albuginea (see Figure 12.1.5-3B). Penetrating towel clamps may be secured to the apical ligament without injury to the penis.
Although the surgeon is principally concerned with the structural integrity of the reproductive tract and its surgical restoration, any surgery of the reproductive tract should, wherever possible, be preceded by thorough breeding soundness examination, including assessment of semen quality, to preclude other, nonsurgical problems that would persist after surgical intervention.
Castration
CASTRATION OF CALVES AND SMALL RUMINANTS
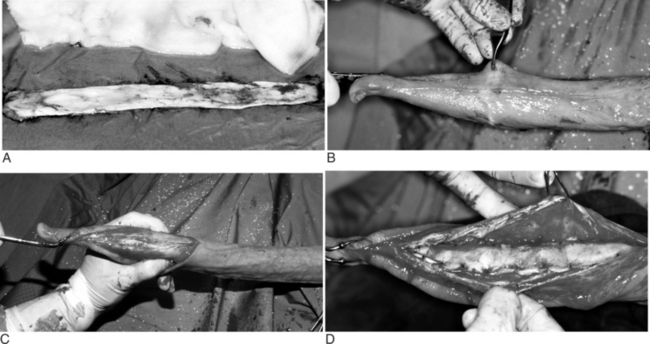
For animals less than 150 kg, the distal portion of the scrotum is grasped and pulled distally, thus displacing the testes proximally (Figure 12.1.1-1). The distal third of the scrotum is excised to expose the testes (Figure 12.1.1-2). Traction is applied to each testis, and the spermatic cord is freed by stripping the fascia proximally (Figure 12.1.1-3). At this point, the cord can be ligated and transected, emasculated, or stretched until the vasculature ruptures. The latter technique results in vasospasm, which is usually adequate hemostasis for smaller animals but could damage the inguinal ring in lambs and lead to a hernia. For this reason, use of a technique that closes (use of emasculator [Figure 12.1.1-4] or ligation) the spermatic cord is preferable. The wound is usually left open to heal by second intention.
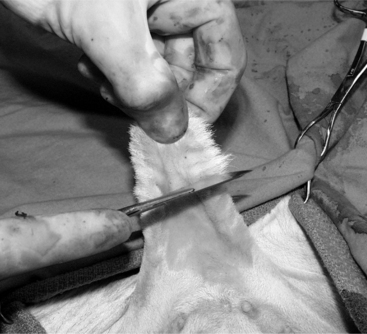
Figure 12.1.1-1 The distal portion of the scrotum of a bull calf in dorsal recumbency is grasped and pulled distally, thus displacing the testes proximally.
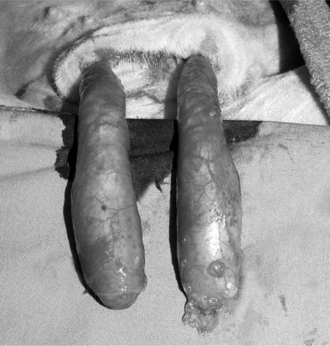
Figure 12.1.1-3 The spermatic cord is exposed by stripping the fascia proximally and applying traction to each testis.
Another surgical option for calves is to approach the testes by making a vertical incision in the lateral wall of the scrotum with a Newberry knife (Figure 4.4-6A). The Newberry knife blade is placed through the middle of the scrotum (Figure 4.4-6A) and rapidly pulled distally, thus making a cranial and caudal flap of the scrotal skin (Figure 4.4-6A). The same flaps can also be created with a scalpel. The testes are excised as mentioned previously, either with traction, an emasculator, or ligation and excision. The wound is typically left open.
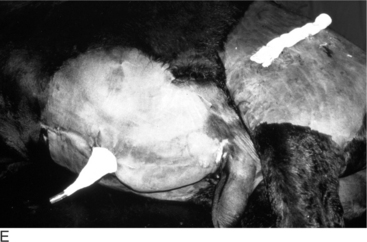
Older animals are occasionally castrated, with the best results obtained when they are restrained in chutes or on a tilt table. Local anesthetic in the scrotal skin and spermatic cord is appropriate. The operator’s preferences dictate the surgical approach. The distal scrotum can be removed (Figure 12.1.1-5), or vertical incisions can be made on either side of the median raphe. Regardless, the testis is identified and freed from its surrounding fascia with blunt dissection. Once isolated, the spermatic cord is ligated and transected or emasculated. Generally, the vaginal tunic is not incised and is removed en bloc with the testis. If the vaginal tunic is incised, the testis and spermatic cord are ligated (or emasculated). Some prefer to split the spermatic vessels away from the pampiniform plexus and ligate (or emasculate) these structures separately. Regardless, the cremaster muscle is ligated with the vaginal tunic, and both are transected approximately 2 cm distal to the ligations. Transfixation sutures help prevent ligature slippage. If primary closure is to be performed, at least a partial resection of the redundant scrotal skin is necessary to minimize dead space. The subcutaneous tissues are closed with a pursestring-type suture (superficial and deep bites) using an absorbable suture material. A subcuticular or skin closure should be done with an absorbable suture if primary closure is elected.
UNILATERAL ORCHIDECTOMY
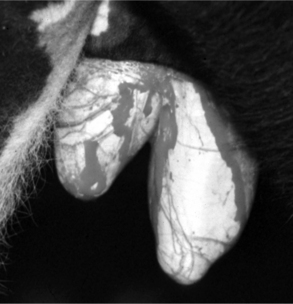
Injury or illness that involves one testis may detrimentally affect the contralateral tests to the extent that removal of the affected testis provides the best option for returning the bull or ram to fertility. This may be the case in unilateral hydrocele, hematocele, testicular tumor, epididymitis, abscess, varicocele (Figure 12.1.1-6A) or other conditions. In such cases the contralateral testis may be compromised by pressure or increased local temperature. Careful removal of one testis results in preservation of the remaining testis, and some compensatory hypertrophy and increased sperm production by the remaining testis may be expected.

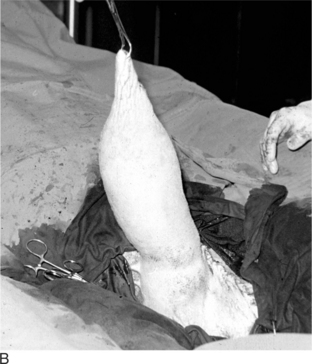
Figure 12.1.1-6 A, Mature ram with unilateral varicocele. Note the enlarged asymmetric scrotum. B, Intraoperative view before resection.
(Courtesy of Dr. Mary Smith; Cornell University.)
The surgical procedure for unilateral orchidectomy is as follows. The animal is restrained or anesthetized in lateral recumbency. The upper hind limb is abducted and secured in a fixed position; the scrotum may be held by a towel clamp to facilitate preparation (Figure 12.1.1-6B). After preparation of the surgical site, a vertical incision is made on the lateral aspect of the affected side approximately the entire length of the testis. The initial dissection extends the skin incision through the scrotal fascia, and the testis is bluntly dissected within the vaginal tunic. The vaginal tunic is incised the length of the testis, and the spermatic cord isolated, double-ligated, and transected or emasculated. The tunic can be transected and oversewn with an absorbable suture material. It is usually necessary to remove excess scrotal skin. This will help minimize dead space and prevent postoperative seroma formation. The scrotal fascia is closed by using a continuous pattern with an absorbable suture. The subcutaneous tissues and skin are closed routinely. Studies have shown that semen quality returned to normal 22 days after unilateral castration in normal bulls. There are other case reports of successful return to production following surgery in several animals with unilateral disease. Currently, surgery is recommended for valuable animals with nonheritable unilateral disorders other than herniation.
BLOODLESS CASTRATION
Several techniques for castrating farm animals without performing a surgical incision are available. These so-called “bloodless techniques” create ischemia of the testis with subsequent atrophy or necrosis. In very young animals, the most common technique is to use an elastic band. The band and applicator pliers should be soaked in disinfectant before use. Once the animal is restrained, the band is applied around the neck of the scrotum by using the pliers (Figure 12.1.1-7A and B). Other commercially available tools are designed to place heavy-walled latex tubing around the neck of the scrotum in older animals. The scrotum and testes usually slough within 3 weeks of band application. This technique is used quite commonly in small ruminants less than one month of age. Ambulatory clinicians at our institution have been successful with using a callicrate bander* in bulls up to 400 kg (Figure 12.1.1-8). Rare failure has been associated with band breakage. Older, small ruminants should be handled the same as older calves with sedation and surgery; however, one should always be mindful of small ruminant sensitivity to lidocaine. The callicrate bander has been used successfully on older goats after the long hair was clipped from the neck of the scrotum.
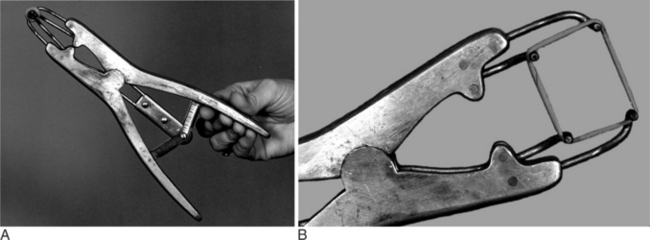
Figure 12.1.1-7 A, Elastrator used to apply elastic bands when castrating a young kid. B, Close-up view.
Another bloodless technique is to use a Burdizzo emasculatome (Figure 12.1.1-9) to crush the spermatic cord within the scrotum. After this, the testes atrophy but usually do not slough. This instrument is best used by crushing a portion of the scrotum while holding the spermatic cord over the side that is crushed. One can crush the spermatic cord twice while manipulating the cord within the scrotum. These crushes should be staggered without crossing the midline; so no pressure is applied across the entire scrotum, thus minimizing the risk of impairing vascular supply. All animals treated with nonsurgical castration techniques should receive tetanus prophylaxis (ideally several weeks before the procedure) because tetanus acquisition is one of the complications of this procedure.
12.1.2 Testicular Biopsy
Robert O. Gilbert and Susan L. Fubini
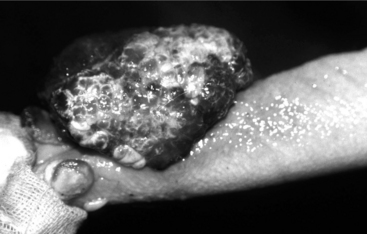
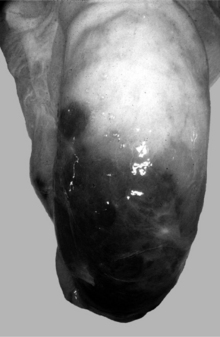
Recently, testicular biopsy was performed on six normal bulls to determine whether this would be a useful diagnostic tool for bulls with infertility. The investigators used a 15.2-cm 14-gauge biopsy needle placed through a stab incision in the skin. They determined that there were no long-term changes in semen quality over the course of the 90-day study. Therefore testicular biopsy with histopathological examination may be considered for cases of infertility if time and economic constraints preclude a wait-and-see approach for determining the future breeding ability of a particular bull. It is important to obtain the tissue sample from the lateral cranial aspect of the proximal (dorsal) area of the testis where blood vessels are relatively sparse to prevent excessive hemorrhage and infarction. Severe hemorrhage may necessitate removal of the testis (Figure 12.1.2-1). Cultures and sensitivity should be used to assess a testicular condition associated with an inflammatory or infectious process.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree