Chapter 10 Surgery of the Bovine Digestive System
10.1 Surgical Diseases of the Oral Cavity
Many surgical diseases can interfere with an animal’s ability to prehend and transfer food material to the esophagus. The cause of dysphagia can be a congenital abnormality or diseases acquired through pain and/or mechanical obstruction. Of course, pain itself can prevent an animal from eating or drinking (e.g., severe oral inflammations, mandibular fracture, glossitis, foreign body penetration, temporohyoid arthropathy, etc.). Mechanical causes of dysphagia include a foreign body, anatomical defects such as cleft palate, or peri-pharyngeal masses such as neoplasia and abscess. Although this chapter focuses on surgical diseases, when evaluating an animal with dysphagia, one should consider a variety of centrally mediated neuromuscular disorders such as listeriosis and rabies. Peripheral neurological diseases such as neuropathy of the lingual and hypoglossal nerves should also be considered. Probably the most common cause of muscular disorders is white muscle disease. The following sections propose a diagnostic and therapeutic approach to surgical diseases of the oral cavity.
Anatomical Considerations
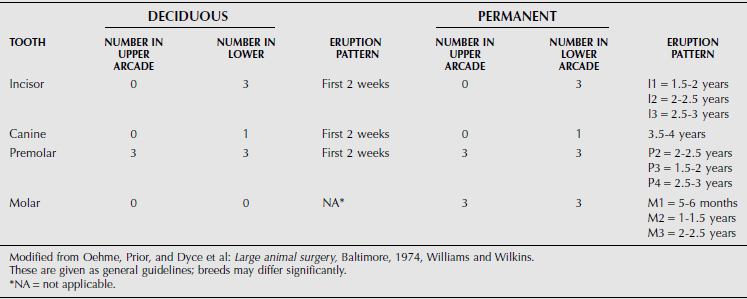
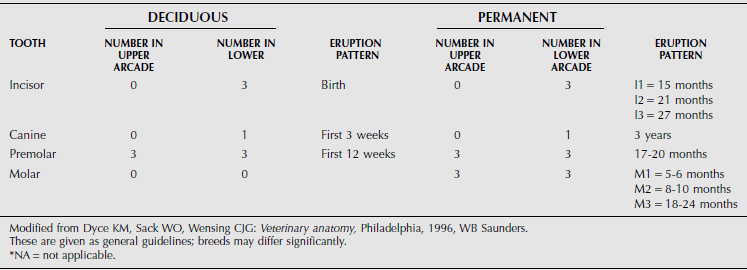
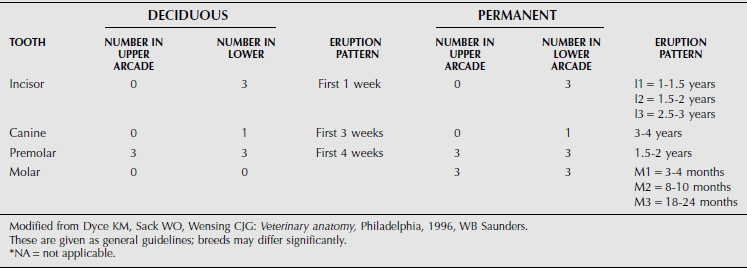
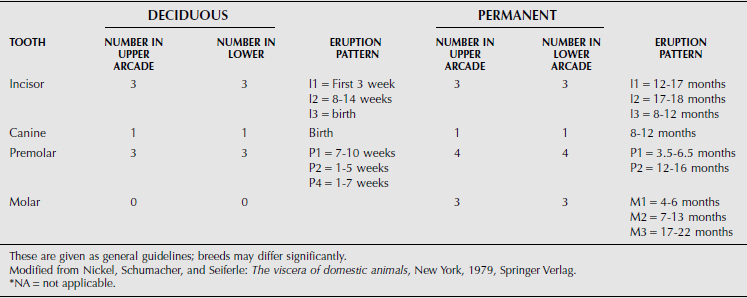
The dental formula of farm animals is summarized in Tables 10.1-1 through 10.1-4, but breeds may differ significantly. The canine tooth is also called the fourth or corner incisor. A significant anatomical characteristic is the shallow depth of the alveolar socket and associated tooth root in cattle. This leads to dental attrition in older cattle more readily than in horses but also facilitates tooth excision in advance of dental disease.
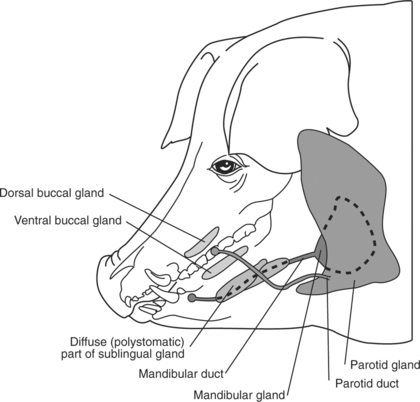
The large quantity of salivary glands in ruminants (Figure 10.1-1) and swine (Figure 10.1-2) contribute to large amounts of saliva being produced, estimated to be as much as 100 liters per day in adult cattle. The left and right parotid glands are located ventral to the ear, extend along the caudal border of the mandible, and drain into the mouth by a single large duct (i.e., the parotid or Stenson’s duct). The parotid duct continues rostrally along the ventral border of the mandible following the rostral aspect of the masseter muscle and finally opening in the caudal aspect of the mouth at the level of the second to last cheek tooth. The left and right mandibular glands are more axial than the parotid glands but also more ventral they are and centered on the angle of the jaw (mandible). Each gland drains into the mouth by its own single duct. These mandibular ducts extend rostrally submucosally and open on their respective sublingual caruncle on either side of the frenulum of the tongue. The left and right sublingual salivary glands contain two parts: a monostomatic and a polystomatic. The polystomatic glands lie on either side of the tongue on the floor of the mouth and drain to many stomas beside the frenulum. The left and right monostomatic glands are located rostral to their respective ipsilateral polystomatic gland and drain into the mouth through a single duct alongside—or joining—the mandibular duct. Many other small salivary glands exist in various locations of the oral cavity.
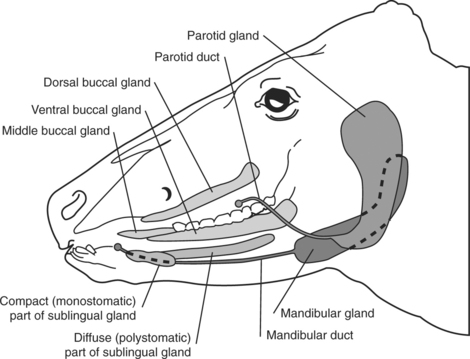
Figure 10.1-1 Schema summarizing the distribution of salivary glands in ruminants.
(Redrawn from Dyce KM, Sack WO, Wensing CJG: The digestive system. In Veterinary anatomy, Philadelphia, 1996, WB Saunders.)
Diagnosis and Treatment
LACERATIONS
Oral lacerations in cattle are associated with the same indiscriminate eating habit which results in traumatic reticulopericarditis. Lacerations are more common in calves because of their oral prehension and suckling habits on objects in their environment such as barbed wire, needles, and thorns. The lacerations may involve the lips, buccal membranes, and the tongue. Animals usually present with excess salivation, which may be mixed with blood, decreased appetite, and various degrees of dysphagia, depending on the severity of the laceration. The animal’s tongue often protudes past its lips.
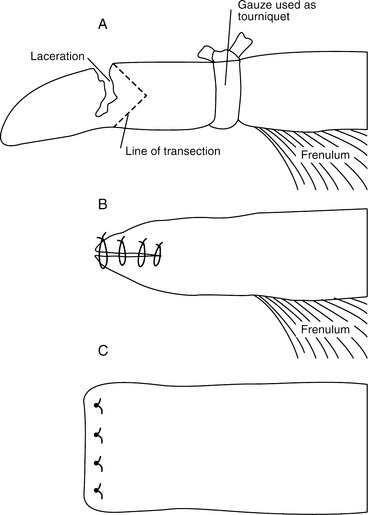
Severe tongue lacerations sometimes require a partial glossectomy. Because of the tongue’s crucial role in prehension of food, as much of the tongue as possible should be preserved. In preparation for surgery The animal is anesthetized and placed in lateral recumbency. A tourniquet (made of rolled gauze) is applied proximal to the intended transection site. The tongue is transected so that the dorsal and ventral aspects protrude beyond the center (Figure 10.1-3A). The ventral and dorsal aspects are sutured together with an interrupted horizontal mattress pattern with a no.-1 or no.-2 absorbable sutures (Figure 10.1-3B and C). The animal should receive systemic antibiotics postoperatively and should be fed a soft diet (not pasture) for best results.
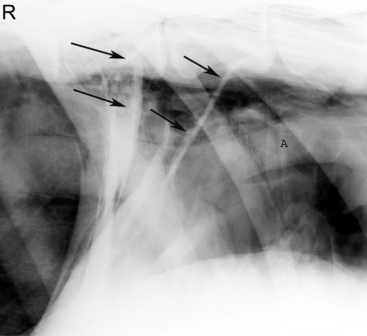
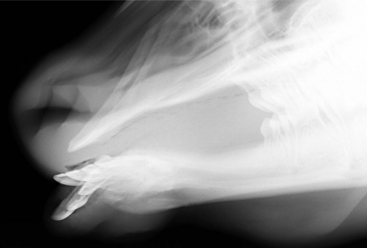
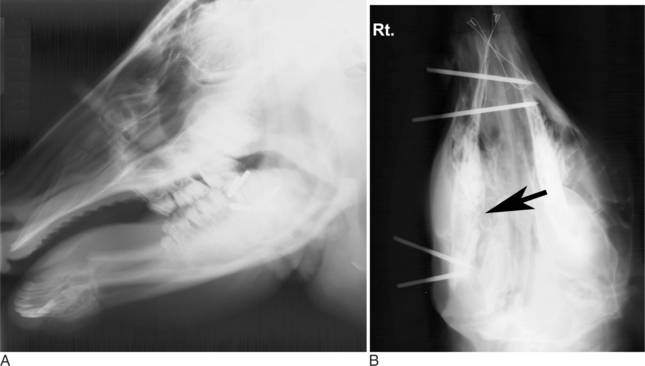
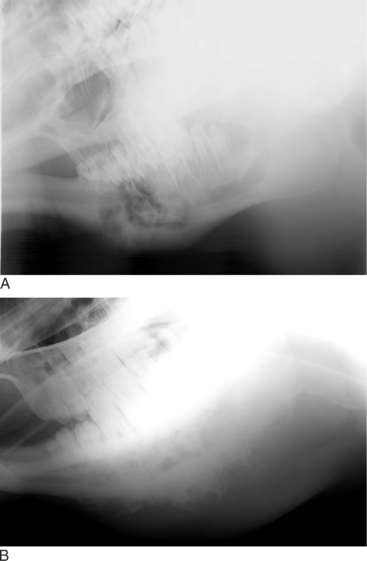
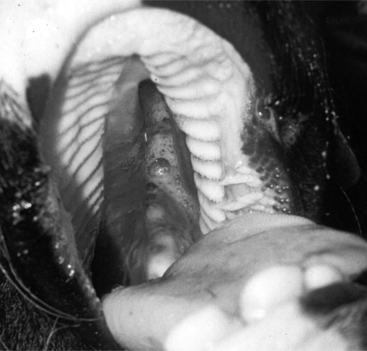
Oropharyngeal trauma and subsequent retropharyngeal cellulitis and dysphagia can occur after improper administration of medication with a balling gun. Animals are presented because they become anorectic and have an associated decrease in milk production (when relevant). On examination, they have varying degrees of cervical swelling and associated signs of infections—elevated temperature, leukocytosis, and hyperfibrinogenesis. The cervical swelling may interfere with respiration (see disorders of the nasopharynx, Section 9.2.1.1.3); the animal will extend its head and neck while trying to straighten their upper airway (Figure 10.1-4). A foul-smelling odor indicative of necrotic tissue may originate from the mouth. Endoscopic, or open-mouthed, examination of the nasopharynx and oropharynx will reveal the laceration and/or abscess (Figure 10.1-5). The cervical area is swollen, and crepitus can sometimes be palpated if the area is not too severely distended. Ultrasonographic evaluation will reveal pockets of fluids in the subcutaneous tissue of the proximal cervical area. Radiographic evaluation will reveal air and fluids in the cervical area (Figure 10.1-6). These animals may aspirate feed and saliva and develop signs of lower airway disease. Therefore the lower airway should be evaluated for signs of mediastinitis (Figure 10.1-7) and aspiration pneumonia.
The treatment principle is to limit the extension of the cellulitis with appropriate parenteral antimicrobials and surgical drainage. If cellulitis is not controlled, it will proceed alongside the trachea and may result in septic mediastinitis (see Figure 10.1-7). Therefore if there is significant accumulation of fluid and feed material in the cervical area, the accumulated fluid is surgically drained under general anesthesia. See disorders of the nasopharynx (Section 9.2.1.1.3) for a description of this procedure.
SELF-SUCKLING
Self-suckling is most commonly treated by using a nasal ring with a burr (Figure 10.1-8) or nasal flap and individual housing. If these more conservative treatments are not successful, a partial glossectomy can be considered.
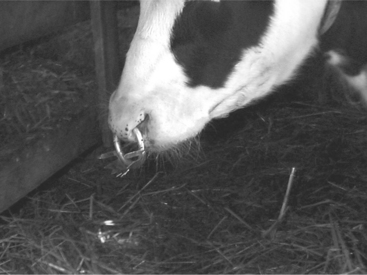
Figure 10.1-8 A, Weaning ring used to prevent self-suckling.
(Courtesy of Dr. Lorin Warnick, Cornell University.)
Partial Glossectomy
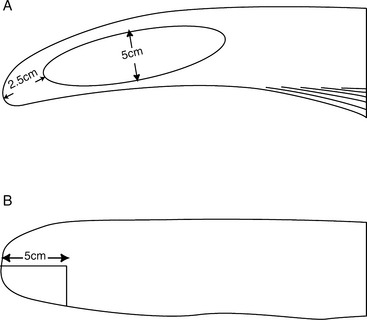
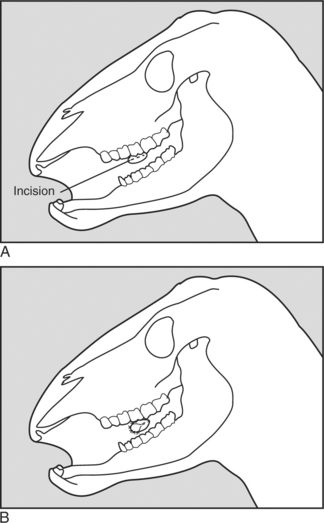
Two surgical techniques have been created to perform a partial glossectomy to prevent self-suckling in animals. The techniques are performed with sedation and local infiltration of lidocaine or general anesthesia. Both techniques alter the tongue’s contour to prevent the animal from forming a U-shaped tongue for suckling. For the ventral glossectomy technique, an elliptical incision is made that is approximately 5 cm at its widest part and starts rostral to the frenulum attachment on the tongue and extends rostrally 2.5 cm caudal to the tip of the tongue (Figure 10.1-9A). Each side of the ellipse is incised at an angle toward the midline to facilitate closing the defect, as shown in Figure 10.1-3B. The lateral glossectomy technique removes half of the tip of the tongue (Figure 10.1-9B). Again, the incision is extended at an angle to facilitate closing the tongue similar to what is shown in Figure 10.1-3B, except in a different plane.
SALIVARY GLANDS
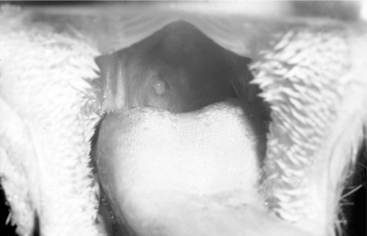
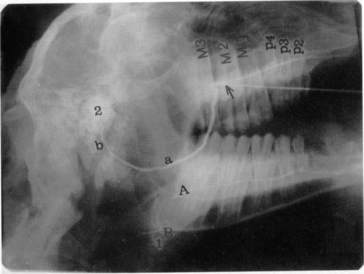
Diagnosis of these various diseases is made by physical examination and salivary diagnostic imaging, such as ultrasound exam or sialogram. Palpating a soft fluctuant swelling within the confines of a parotid duct (Figure 10.1-10) strongly suggests an obstructed duct with a secondary distension of the duct. An example of a normal sialogram is shown in Figure 10.1-11.

Figure 10.1-10 A soft fluctuant swelling can be palpated within the confines of a salivary duct in this young goat.
(Courtesy of Dr. Mary Smith, Cornell University.)
Many surgical options are available for treating an obstructed duct or salivary gland fistula. Congenital salivary duct obstruction can be left untreated and simply be monitored. The salivary function loss in a unilateral case is insignificant and results mainly in a cosmetic defect.
The duct proximal to the obstruction can be marsupialized to the oral cavity. This is technically difficult because the duct’s unobstructed section is always more caudal than the anatomical opening. The marsupialization is done as follows. A longitudinal incision is made in the oral cavity at the level of the distended duct (Figure 10.1-12A). The incision is extended to the axial wall of the parotid duct in the same plane. Saliva will leak out into the incision. The incision is enlarged so the stoma created is 1 to 1.5 cm. The oral mucosa is sutured to the parotid duct mucosa with a simple interrupted pattern of absorbable monofilament suture material of appropriate size (2-0 or 3-0) (Figure 10.1-12B). A size-5 to size-8 French polyethylene catheter should be passed through the newly formed stoma and sutured to the buccal mucosa to prevent unwanted closure. The catheter is removed 7 to 10 days later.
The gland may be injected with a caustic agent to destroy all secreting cells until the fistula resolves and heals. Use of 10 to 15 ml of Lugol’s iodine or up to 35 ml of 10% buffered formalin (check with local regulatory veterinarian) injected through a catheter placed into the duct for this procedure has been reported. The duct must be held closed for a few minutes to achieve diffusion of the caustic agent throughout the gland. Posttreatment glandular and periglandular swelling may require an antiinflammatory agent such as acetylsalicylic acid or flunixin meglumine.
FRACTURES
In calves, common fractures involve the rostral aspect of one mandible (Figure 10.1-13) or along the mandibular symphysis. If the fracture is minimally displaced, it often heals without treatment. Some calves fracture the rostral aspect of both mandibles in the interdental space, thus resulting in significant displacement that requires treatment (Figure 10.1-14A). In adult ruminants or in any cases in which significant displacement is present, reduction and immobilization is indicated. In all cases, stabilization reduces pain and allows eating to be resumed more quickly.
SURGICAL OPTIONS FOR FRACTURES
Surgical options to reduce and immobilize a fracture of the mandible, maxilla or incisive bone are acrylic, wires, U-bar, Kirchner apparatus, and internal plates. The techniques are described in the following discussions. In general, using the simplest treatment method is far better. For most (if not all) fractures of the rostral mandible, a wire applied in a figure-eight fashion around incisors on either side of the fracture is sufficient. A wire cannot be appropriately tightened around the incisor and canine teeth of some animals because the teeth are too short, so an acrylic bridge or cap is added. This applies to the majority of ruminant orofacial fractures.
Figure-Eight Wiring
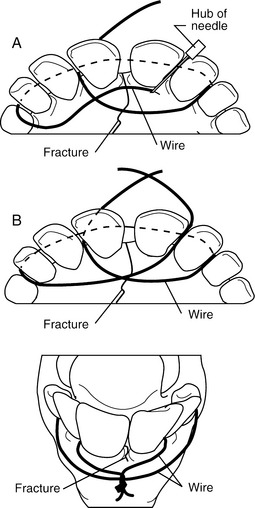
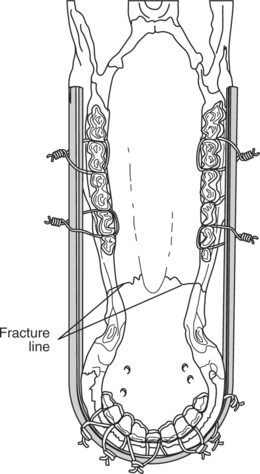
This technique consists of placing an orthopedic wire (1-1.2 mm) around the base of one or more teeth on either side of the fracture in a figure-eight pattern (Figures 10.1-14B and 10.1-15A-B). A 14-gauge needle can be placed to help pass the wire between two teeth (Figure 10.1-15A). Alternatively, a drill can make a canal through which the wire is passed. The knot is twisted and secured on the rostral aspect of the mandible. If the fracture extends caudal to the four incisors (canine teeth) or extends into the interdental space, the wire is secured to the first cheek tooth or a canal is drilled into the bone (incisive or mandibular) in the interdental space (Figure 10.1-16) between the incisors rostral and caudal to the fracture.
Acrylic
This is normally used to provide additional stability to a fixation, generally a figure-eight wire. Although dental acrylics are available, for economic reasons the acrylic* used to secure blocks on cattle hooves is adequate. Because of the exothermic properties of this nondental acrylic, a layer of petroleum gel is applied to the buccal mucosa to protect the soft tissue before the acrylic is applied.
Screw Fixation
Screws should be applied in a lag fashion at approximately 90 degrees to the fracture plane. Using a drill guide to protect the soft tissue, the surgeon over drills the proximal fragment through stab incisions with an appropriate sized drill bit (4.5 mm or 5.5 mm, respectively, for cortical screws of these diameters). An insert is placed, and the distal far fragment is drilled with a 3.5- or 4-mm drill bit, respectively, for the previously mentioned screw diameters. If 6.5-mm partially threaded cancellous screws are used, a 3.5- or 4-mm drill bit is used through the proximal and distal fragment. The drill hole length is measured, and a screw that length minus the fracture gap is measured. After a tap the same size as the screw diameter is used, the appropriate length screw is inserted and tightened. In young animals, a washer under the screw head may be needed. Screws may be used in combination with figure-eight wires using tension band principles.
U-bar
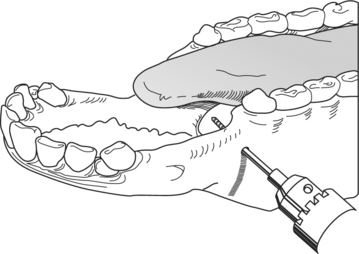
This technique is rarely used because of its difficulty in application. A smooth, round 1/4 inch (6.35 mm) rod is bent into the shape of a “U.” Holes are drilled into this rod to allow passage of orthopedic wires. The U-bar is inserted on the outside of the mandible or maxilla (Figure 10.1-17). Orthopedic wires are passed around the base of various incisors and cheek teeth and secured to perforations in the U-bar. This is readily done around the incisors, but is particularly difficult when securing the wire to the cheek teeth. Because ruminants have a small commissure, one needs to pass a 14-gauge needle through the cheek and guide it between the teeth of interest. The wire is placed through perforations in the U-bar after it has been secured between the cheek teeth. A small buccostomy that avoids the commissures of the lips is sometimes needed, but it increases the technique’s morbidity rate.
Kirschner-Ehmer
The primary indication for this technique is a fracture of the mandible caudal to the symphysis. Type I or II immobilization can be used. With general anesthesia, the animal is placed in dorsal recumbency unless additional fixation is required through the oral cavity; in the latter case, lateral recumbency is selected to keep the fractured side uppermost. The goal is to place two intramedullary (IM) pins (9.53 or 6.35 mm) on each side of the fracture through stab incisions placed on the lateral and ventral aspect of the mandible. After the stab incisions, the soft tissue is retracted with a curved hemostat or drill guide to protect the soft tissue overlying the mandible. Using a smaller drill bit than the IM pins, the surgeon drills a hole into the lateral and ventral aspect of the fractured mandible (type I fixator) on the rostral end of the fracture. The drill hole must be placed in the ventral third of the mandible to avoid tooth roots. Radiographic guidance is helpful in preventing later complications. An IM pin, preferably a positive profile pin, is then placed. A second IM pin is placed at a converging angle. The procedure is repeated on the caudal mandibular fragment. The pins are stabilized by a connecting rod made from a 2.5-cm scavenger hose filled with acrylic (see Figure 10.1-14B). The connecting rods are wrapped with bandage material* to fill the defect between the connecting rod and mandible. This prevents the object from inadvertently violating this space and causing disruption of the repair. Alternatively, a commercially made connecting rod can be used, but this is usually not economically justifiable. In all cases, the pins must be cut close to the connecting rod and a rubber hose or other protective material is used to cover their sharp ends.
A pinless external fixator† was recently introduced. The system consists of pinless clamps in different sizes and geometries. The clamps are applied to the bone cortex (without penetrating the medullary cavity) and fixed in place by tightening a nut. The universal clamps connect to a connector bar, thus creating an external fixator. The advantages of the technique are that it avoids penetrating the medullary cavity and damaging the tooth root as well as its ease of application and minimal surgical time. The pinless system’s disadvantage in comparison to the previously described techniques is its increased cost.
COMPLICATIONS
Osteomyelitis, sequestration, and tooth root abscess are treated with surgical debridement and curettage under general anesthesia. Systemic antibiotics are needed for osteomyelitis cases. Tooth root abscesses are treated by excision as described earlier in this chapter.
Osteomyelitis
A sporadic cause of mandibular or maxillary osteomyelitis is actinomycosis infection (lumpy jaw) in cattle and sheep. Pathologically, this results from an opportunistic infection by Actinomyces bovis after trauma. Animals present with a painful bony swelling that progresses if untreated and eventually shows an eroded ulcerated area devoid of skin and progressively increasing facial deformation (Figure 10.1-18). After biopsy of the mass, the diagnosis is made by gram stain evaluation where sulfur granules are observed. The sulfur granules of actinomycosis are large and oval or horseshoe-shaped. There are also a number of gram-positive, filamentous or short rodlike hyphae beneath clubs. The radiographic appearance of this lesion is typical: an enlarging osseous mass with a honeycomb appearance (Figure 10.1-19). These masses have reportedly resolved solely through medical treatment consisting of penicillin G (22,000 IU/kg sid) and isoniazid (10-20 mg/kg sid), both for 30 days. In addition, sodium iodine is administered (30g/450 kg, IV) every 2 to 3 days until signs of toxicity (i.e., iodism—dry skin, head, neck, and shoulder) are noted.
MISCELLANEOUS
Neoplasia
Chapter 3 discusses neoplasia. Tumors of dental origin (odontoma and ameloblastoma or adamantinoma) have been reported in cattle and sheep. In addition, hamartomas are sometimes seen (Figure 10.1-21). The term hamartoma refers to a mass composed of normal cellular elements that originates from the tissue where it is found. Unlike normal tissue, hamartomas are poorly organized and are believed to be developmental abnormalities rather than true neoplasms. They have been seen on the maxilla but are more common on the rostral aspect of the lower jaw. Their position interferes with mastication. These are usually seen in young animals (<3 years of age). Osteosarcoma and lymphosarcoma should be considered if a hard swelling on the mandible or maxilla is detected on physical examination. It can be differentiated from Actinomycosis because of the latter’s characteristic radiographic pattern and typical presence of skin ulceration and pyogenic granulomas. In all of the aforementioned cases, the diagnosis is confirmed by histopathological evaluation of a biopsy.
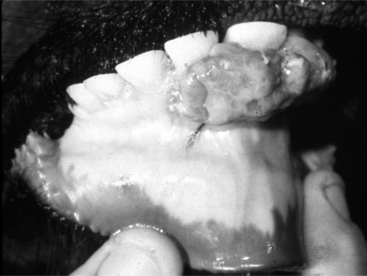
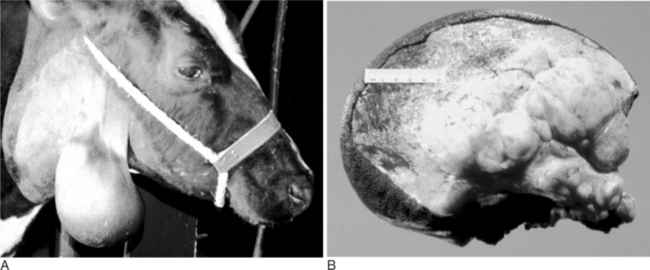
An unusual but characteristic swelling seen in young (1 to 2 years) cattle is a neurofibroma (see Chapter 3). Typically, it consists of a mucocutaneous lesion(s) in young cattle around the head and neck (Figure 10.1-22A). They present as nonpainful masses that enlarge progressively in size. The mass results in a cosmetic defect and gets traumatized because of its location and size. It has been suggested that this is a heritable defect caused by a mutation at the bovine neurofibromatosis type 1 locus.
Ultrasound examination reveals a multilobular appearance to the mass, and when cut in cross-section, reveals the typical appearance as shown in Figure 10.1-22B. Needle aspiration fails to yield purulent material. The diagnosis can be confirmed by biopsy and is usually treated by surgical excision. The author has treated such lesions by excision, as biological progression in untreated patients can cause clinical problems, especially in periorbital lesions.
The animal is anesthetized and placed in lateral recumbency. A fusiform incision is made around the base of the mass. The neck of the mass is identified and isolated via blunt dissection and ligation of transected vessels. Following transaction closure is routine, except in lesions involving the eyelid where careful reconstruction is required. Recurrences have not been encountered in our limited experience but are biologically possible.
Amstuz HE. Dental problems. Modern Vet Pract. 1979;60:639-640.
Bent JP3rd, Klippert FN, Smith RJ. Management of congenital buccopharyngeal membrane. Cleft Palate Craniofac J. 1997;34:538-541.
Davidson HP, Rebhun WC, Habel RE. Pharyngeal trauma in cattle. Cornell Vet. 1981;71:15-25.
Dehghani SN, Tadjalli M, Masoumzadeh MH. Sialography of sheep parotid and mandibular salivary glands. Res Vet Sci. 2000;68:3-7.
Dyce KM, Sack WO, Wensing CJG. The head and ventral neck of ruminants. In: Dyce KM, Sack WO, Wensing CJG, editors. Veterinary anatomy. Philadelphia: WB Saunders, 1996.
Henninger RW, Beard WL, Schneider RK, Bramlage LR, Burkhardt HA. Fractures of the rostral portion of the mandible and maxilla in horses: 89 cases (1979-1997). J Am Vet Med Assoc. 1999;214:1648-1652.
Hofmeyr CFB. The digestive system. In: Oehme FW, Prier JE, editors. Large animal surgery. Baltimore: Williams and Wilkins, 1974.
Horney FD, Wallace CE. Surgery of the bovine digestive tract. In: Jennings PB, editor. The practice of large animal surgery. Philadelphia: WB Saunders, 1984.
Kubo M, Osada M, Konno S. A histological and ultrastructural comparison of the sulfur granule of the actinomycosis and actinobacillosis. Natl Inst Anim Health Q (Tokyo). 1980;20:53-59.
Lischer CJ, Fluri E, Kaser-Holtz B, et al. Pinless external fixation of mandibular fracture in cattle. Vet Surg. 1997;16:14-19.
Parks AH, Baskett SA. Salivary gland disease. In: Robinson NE, editor. Current therapy in equine medicine. Philadelphia: WB Saunders, 1997.
Plumlee KH, Haynes JS, Kersting KW, Thompson JR. Osteosarcoma in a cow. J Am Vet Med Assoc. 1993;202:95-96.
Rebhun WC. Infectious diseases of the gastrointestinal tract. In: Rebhun WC, editor. Diseases of dairy cattle. Baltimore: Williams and Wilkins, 1995.
Sartin EA, Doran SE, Riddell MG, Herrera GA, Tennyson GS, D’Andrea G, Whitley RD, Collins FS. Characterization of naturally occurring cutaneous neurofibromatosis in Holstein cattle: a disorder resembling neurofibromatosis type 1 in humans. Am J Pathol. 1994;145:1168-1174.
Schmotzer WB, Hultgren BD, Huber MJ, et al. Chemical involution of the equine parotid salivary gland. Vet Surg. 1991;20:128-132.
Smoak IW, Hudson LC. Persistent oropharyngeal membrane in a Hereford calf. Vet Pathol. 1996;33:80-82.
Singh JIT, Singh AP, Patil DB. The digestive system. In: Tyagi RPS, Singh JIT, editors. Ruminant surgery. Shandara: CBS, 1993.
St-Jean G, Basaraba RJ, Kennedy GA, Anderson DE. Maxillary lymphosarcoma in a cow. Can Vet J. 1994;35:56-57.
Wiggs RB, Lobprise HB. Acute and chronic alveolitis/osteomyelitis (“lumpy jaw”) in small exotic ruminants. J Vet Dent. 1994;11:106-109.
Wilson RB. Gingival vascular hamartoma in three calves. J Vet Diagn Invest. 1990;2:338-339.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree