Chapter 56 Stomach
Structure and Function
Functional Anatomy
The stomach acts as a reservoir to control the size and rate of passage of ingesta into the small intestine, to initiate the digestion of protein and fat, and to facilitate the absorption of vitamins and minerals (see Chapter 1)
The stomach is composed of five anatomic regions: cardia, fundus, corpus, antrum, and pylorus (Figure 56-1). The fundus and body expand greatly to accommodate ingesta and to regulate the emptying of liquids. The antrum grinds food into smaller particles (<2 mm) that are sieved into the duodenum. The gastroesophageal sphincter prevents reflux of gastric fluid into the esophagus, and the pyloric sphincter controls emptying into the small intestine.
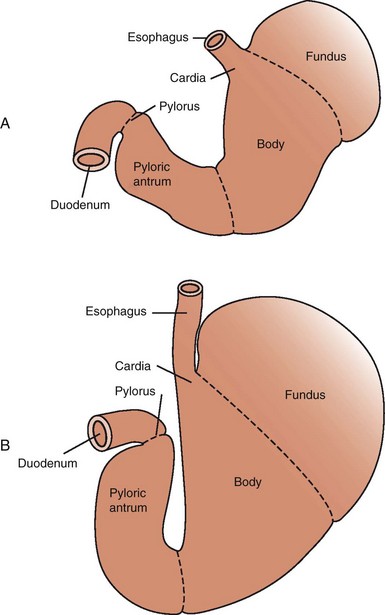
Figure 56-1 Gastric anatomy of the empty (A) and full (B) stomach.
(From Guilford and Strombeck: Strombeck’s Small Animal Gastroenterology, St. Louis, Saunders, 1996, p 239.)
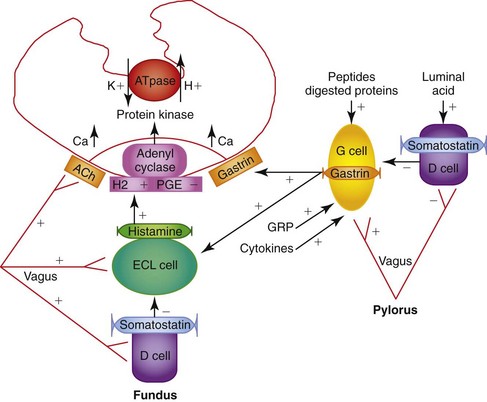
The gastric wall contains a mucosa, submucosa, muscularis externa, and serosa (see Figures 56-2 and 1-3, B). The mucosa has a superficial epithelium, gastric glands, and an innermost layer of smooth muscle (the muscularis mucosa), with fine structure and function varying depending on the gastric region. The mucosa in the cardia and pylorus is thinner and less glandular than in the fundus and body. The mucosa of the body contains mucous neck cells (producing mucous, pepsinogen A, and gastric lipase), parietal cells (producing H+, pepsinogen A, and intrinsic factor), and chief cells (producing pepsinogen A) (Figure 56-2).1–4 A variety of neuroendocrine cells are involved in the regulation of acid secretion and are interspersed between the glands. The predominant cells are enterochromaffin-like and somatostatin-producing cells in the fundus, and gastrin and somatostatin-producing cells in the antrum (see Figure 56-2). Localized small aggregates of lymphoid tissues are frequently observed at the base of the gastric glands. A rich network of blood vessels, lymphatics, and nerves weaves between the gastric glands. Beneath the submucosa are two layers of smooth muscle (circular and longitudinal) that run perpendicular to one another. The serosa is the outermost layer.
Regulation of Acid Secretion
Physiologically, luminal peptides and gastric distention are the primary stimuli for H+ secretion from parietal cells. Pharmacologically, parietal cell acid secretion is regulated by endocrine (gastrin), neurocrine (acetylcholine), and paracrine (histamine) mechanisms.4,5 Somatostatin released in response to gastric pH levels below 3 provides negative feedback and decreases gastrin, histamine, and acid secretion.
Luminal peptides and gastric distention stimulate gastrin secretion from G cells and effect histamine release from enterochromaffin-like cells. Gastrin-releasing peptide and acetylcholine are the primary enteric neurotransmitters regulating gastrin release from antral G cells (Figure 56-3).
Unstimulated acid secretion in dogs and cats is minimal6–8 (e.g., dogs <0.04 mmol/kg0.75/h) and the unstimulated H+/K+-adenosine triphosphatase (ATPase), “the proton pump,” is localized within tubulovesicles in the cytoplasm of parietal cells.4,9 The stimulated H+/K+-ATPase and KCl transporters are incorporated into the parietal cell canalicular membrane and hydrogen ions (derived from the ionization of water within the parietal cells) are transported into the gastric lumen in exchange for K+ by H+/K+-ATPase. Potassium and chloride transporters in the canalicular membrane enable luminal transfer of potassium (for recycling via H+/K+-ATPase) and chloride. Hydroxide combines with CO2, catalyzed by carbonic anhydrase, to form HCO3−, which diffuses into the blood giving rise to the “alkaline tide” phenomenon. Recent studies have expanded our knowledge of ion transport in the parietal cell by identifying KCNQ1 as the primary channel responsible for K+ recycling and establishing the contribution of CFTR (cystic fibrosis transmembrane regulator) and SLC26A9 to the process of chloride secretion.5 Figure 56-4 shows a schematic of ion transport in the parietal cell incorporating these findings. Stimulation results in a rapid increase in fluid and hydrogen ion secretion, with pH rapidly declining to around pH 1. The concentrations of K+ (10 to 20 mmol/L) and Cl− (approximately 120 to 160 mmol/L) in gastric juice are higher than in plasma.
The stomach is protected from gastric acid injury by a functional unit known as the gastric mucosal barrier.10,11 The gastric mucosal barrier comprises tightly opposed epithelial cells coated with a layer of bicarbonate-rich mucus and an abundant mucosal blood supply that delivers bicarbonate, oxygen, and nutrients. Local production of prostaglandin (PGE2) is important in modulating blood flow, bicarbonate secretion, and epithelial cell renewal. When damage occurs, epithelial cells rapidly migrate over superficial mucosal defects aided by the local production of growth factors such as epidermal growth factor.
Evaluating Gastric Secretory Function
As a starting point, fasting gastric pH and serum gastrin can be measured to determine if acid hypersecretion is likely. Ideally, antisecretory therapy should be discontinued for 7 days prior to testing,12 and renal and hepatic function should be monitored as these may be associated with decreased serum gastrin clearance and hypergastrinemia. The broad range of unstimulated (fasting) gastric pH in dogs and cats (from pH 1 to 8) makes definitive statements regarding basal acid production difficult. However, the presence of a gastric pH of less than 3 in the face of a high serum gastrin rules out the possibility of achlorhydria, or mast cell tumor, and raises the possibility of gastrinoma.13,14 Dogs with mast cell tumors and hyperhistaminemia-induced acid hypersecretion have low serum gastrin concentrations,15 whereas dogs with achlorhydria likely have a high gastrin, but a gastric pH greater than 3.
Measurement of serum gastrin following the intravenous infusion of secretin or calcium is used to further investigate the possibility of exogenous gastrin production by pancreatic gastrinomas (Zollinger-Ellison syndrome).12–14 Basenji dogs with gastroenteropathy and diarrhea are reported to have elevated gastrin release in response to secretin stimulation, without evidence of gastrinoma.16 Provocative testing of gastric acid secretion with pentagastrin or bombesin stimulation may be performed to detect achlorhydria in patients with atrophic gastritis, or elevated serum gastrin and gastric pH levels higher than 3, and in those with putative idiopathic small intestinal bacterial overgrowth to determine if achlorhydria is a contributing factor. Pentagastrin-stimulated acid secretion in dogs reaches a peak of 28 mL/kg0.75/h, 4.1 mmol HCl/kg0.75/h, 0.34 mmol K+/kg0.75/h, and 0.09 Na+ mmol/kg0.75/h.6 Sedation with oxymorphone and acepromazine may be a suitable alternative to anesthesia for secretion studies in dogs.16 In cats, acid output (mean ± standard deviation) in response to pentagastrin (8 µg/kg/h) ranges from pH 0.9 to 1.1, with secretion rates (median values) of 1.2 mmol/15 min to 1.4 ± 0.5 mmol/15 min in conscious cats and 1.2 (0.6 to 2.7) mmol/kg0.75/h in anesthetized cats.7
Gastric Motility
Normal gastric motility is the result of the organized interaction of smooth muscle with neural and hormonal stimuli.17,18 The rate of gastric emptying is directly proportional to the difference in pressure between the stomach and the duodenum, and inversely proportional to the resistance to flow across the pylorus. Liquids are emptied more rapidly than solids and the rate of emptying of liquids increases with volume. The emptying rate for digestible solids depends on caloric density. In dogs, digestible solids smaller than 2 mm are emptied into the duodenum, and gastric emptying is modulated via intestinal osmo-, chemo-, and tryptophan receptors. Lipids particularly retard gastric emptying. The release of cholecystokinin in response to fatty and amino acids such as tryptophan, is one factor that slows gastric emptying. Large, undigestible solids are expelled from the stomach in the fasted state by phase III of the migrating motility complex (MMC) in response to the release of motilin.
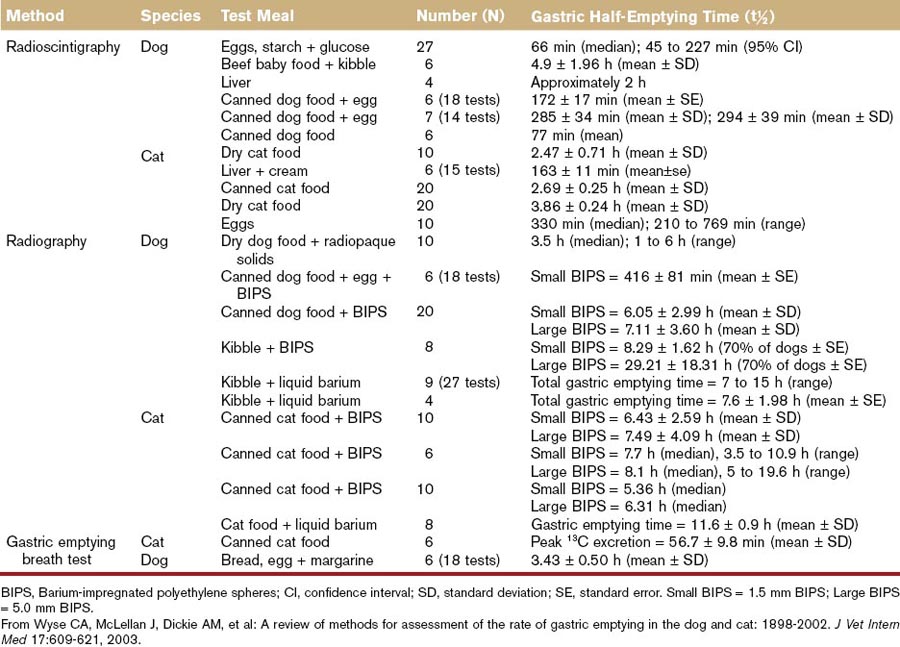
There is wide variation in the rate of gastric emptying reported in healthy dogs and cats that is largely a function of the method used to evaluate it (Table 56-1; see also Table 26-2),19 and in clinical practice delayed gastric emptying is usually suspected when food is retained in the stomach for more than 8 hours. The advent of noninvasive, nonradioactive methods of measuring gastric emptying, such as telemetry capsules,18,20,21 holds the promise of improving our basic understanding of gastric motility in health and disease.
Digestion and Assimilation of Nutrients
The stomach has a limited role in the digestion of proteins, fats, and micronutrients. Pepsin, which hydrolyzes peptide bonds, is secreted as pepsinogen in response to acetylcholine and histamine in parallel with gastric acid secretion. Dog gastric lipase, which digests fat, is secreted in response to gastrin, histamine, prostaglandin E2, and secretin, and lipase secretion parallels the secretion of gastric mucus. Although pepsin is active only at acidic pH, dog gastric lipase remains active in the small intestine and constitutes up to 30% of total lipase secreted over a 3-hour period.22 Although gastric lipase and pepsin are not essential for the assimilation of dietary fat and protein, the entry of peptides and fatty acids into the small intestine likely helps to coordinate gastric emptying and pancreatic secretion.
Intrinsic factor, which is necessary for cobalamin (vitamin B12) absorption, is produced by parietal cells and cells at the base of antral glands in the dog but not the cat.1,23 The importance of gastric intrinsic factor secretion in dogs is questionable as the pancreas is the major site of secretion in both dogs and cats. Gastric acidity may also have an effect on the availability of minerals such as iron and calcium.
Gastric Flora and Immune Surveillance
The concept of the stomach as a sterile place was completely dispelled by the isolation of the gastric bacterium Helicobacter pylori from people in 1983.24 A mixed flora of aerobes and anaerobes (approximately 106 to 107 cfu/mL) is rapidly established soon after birth in dogs,25 and colonization with Helicobacter spp., which are likely acquired from the dam, has been documented as early as 6 weeks of age. The stomach of healthy dogs and cats harbors a diverse spectrum of large, spiral, acid-tolerant Helicobacter species26 that stimulate local and systemic immune responses characterized by lymphoid follicular hyperplasia and seroconversion,27–29 and may play a role in the development of gastritis30,31 and cancer. These host–bacterial interactions are discussed further in Chapter 2 and in “Inflammation” section of Chapter 56. Helicobacter spp. are adapted to life in an acid environment and produce urease, which catalyzes the formation of ammonia from urea to buffer gastric acidity. Other bacterial species, such as Proteus, Streptococcus, and Lactobacillus, cultured from the canine stomach, may transiently increase after a meal or coprophagia. Acid secretion and gastric emptying likely regulate much of this transient flora and bacteria may proliferate in the event of gastric acid hyposecretion because of glandular atrophy or pharmacologic inhibition. From a diagnostic standpoint it is important to recognize that bacteria such as Escherichia coli and Proteus spp. produce urease that can lead to a false-positive test result for Helicobacter spp.
Diagnostic Evaluation
History and Physical Examination
Special attention should be made if the vomiting animal has been treated with nonsteroidal antiinflammatory drugs (NSAIDs), as serious GI ulceration is a well-known side effect of this treatment.1,2 Concurrent weight loss could indicate a low calorie intake, small intestinal involvement (e.g., inflammatory bowel disease [IBD]), or neoplasia. Access to garbage, plants, and cleaners all may be important causes of vomiting. The age and breed is important as young animals are more likely to ingest foreign bodies and older animals more frequently experience gastric cancer. Gastric cancer is more frequently seen in the Belgian Shepherd, Rough Collie, Staffordshire Bull Terrier, Beagle, and Lundehund breeds. The large and giant breeds with deep chest conformation, such as the Great Dane, are at increased risk for gastric dilation volvulus syndrome.
Vomiting is one of the major clinical signs of gastric disorders (see Chapter 23). Descriptions of the vomitus should include the amount, color, consistency, odor, and presence of bile or blood. The relationship of vomiting to food and water consumption should also be noted. Vomiting should also be differentiated from gagging, coughing, dysphagia, and regurgitation, all of which may appear the same for the pet owner, before beginning a medical workup for gastric disease. Nonproductive retching and vomiting in deep-chested dogs may require immediate medical attention for gastric dilation or gastric dilation and volvulus (GDV) syndrome. Persistent vomiting of large volumes of fluid is highly suggestive of GI obstruction, whereas vomiting of digested food 12 hours after feeding suggests delayed gastric emptying because of gastric outflow obstruction or a gastric motility disorder. Associated clinical signs, such as anorexia (nausea caused by gastritis), diarrhea (intestinal involvement), and melena (gastric bleeding), may occur in animals with gastric disease. Box 56-1 lists the most common causes of vomiting, and Table 56-2 lists the diagnostic procedures that are used in the differentiation of the vomiting disorders.
Box 56-1
Common Causes of Vomiting
Table 56-2 Diagnostic Procedures of Value for the Differential Diagnosis of Vomiting
| Procedure | Advantage | Disadvantage |
|---|---|---|
| Survey abdominal radiographs | ||
| Fluoroscopy | ||
| Ultrasonography | ||
| Endoscopy | ||
| Laparoscopy | ||
| Exploratory laparotomy |
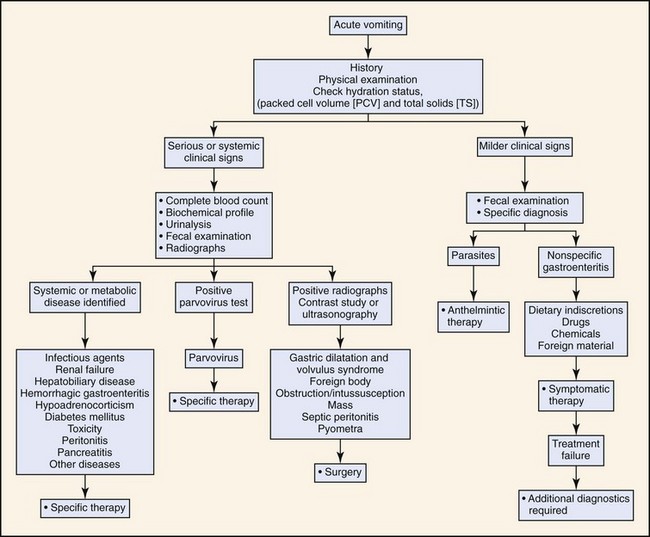
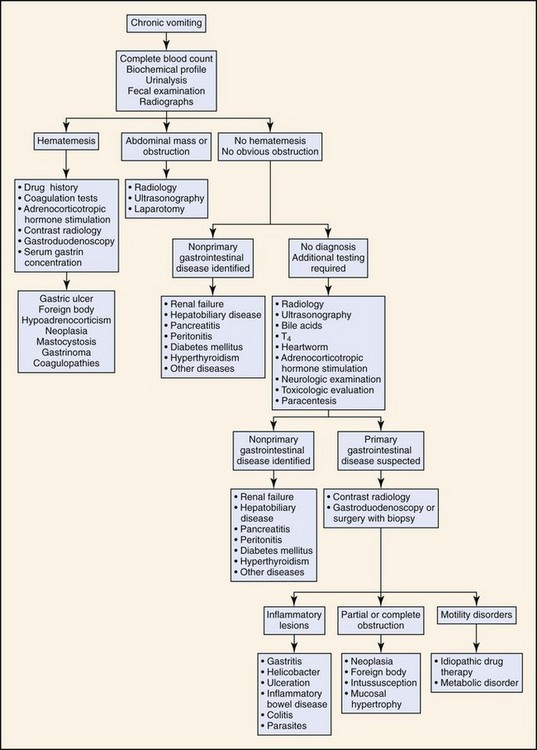
Based on the history and duration of clinical signs, the animal is classified as having either acute or chronic vomiting. The diagnostic approach depends on the duration of the clinical signs and whether the animal has other systemic signs (Figures 56-5 and 56-6). Chronic vomiting is often defined as vomiting that has persisted for 7 to 10 days or when there is no response to initial treatment.
Laboratory Data
Clinicopathologic testing is often warranted when differentiating primary gastric disorders from nongastric disorders. Complete blood cell count, serum chemistry, urinalysis, and fecal flotation should be considered when the patient is persistently vomiting or if there are signs of systemic diseases. Blood and urine samples should be obtained before treatment, which gives the clinician the chance to evaluate the metabolic consequences of the GI disease. Biochemical abnormalities in primary gastric diseases are usually consistent with mild to moderate loss of chloride, potassium, sodium, and bicarbonate. Prerenal azotemia is a result of dehydration. Elevated blood urea nitrogen without concurrently elevated serum creatinine may indicate gastric bleeding. Electrolytes and acid–base status are important parameters if symptomatic therapy is to be optimized in the vomiting animal.3,4
In patients with extensive GI bleeding (melena or hematemesis), an assessment of coagulation is essential. Severe GI bleeding as a consequence of coagulopathy caused by infection with Angiostrongylus vasorum has been reported in dogs. When gastric acid hypersecretion is suspected, as with gastrinoma, gastric secretory testing (e.g., gastrin and fasting gastric pH measurement) should be performed.5 The reader is referred to Chapter 25 for further detail.
Gastric Imaging Techniques
When delayed gastric emptying is suspected, evaluation of gastric emptying is carried out using barium contrast (liquid or mixed with food), barium-impregnated polyethylene spheres, fluoroscopy, nuclear scintigraphy, or 13C-octanoate breath test (see Chapter 26 and Table 56-1).6–8
Ultrasonography is widely used when evaluating GI clinical signs. The gastric fundus is evaluated in left lateral recumbency, and the pylorus is evaluated in right lateral recumbency. Longitudinal and transversal views of stomach are required to measure the size of the organ and thickness of the gastric wall. Ultrasound may be used to assess wall thickness, wall layer identification, wall symmetry, extent of lesions, GI content, and motility. Diseases such as gastric cancer, chronic gastropathy, uremic gastritis, and delayed gastric emptying disorders have been diagnosed by ultrasonography in cats and dogs.9–11 The test is noninvasive and requires little in the way of patient preparation. Ascites may enhance the image of abdominal organs in those patients with ascites. Aerophagia, on the other hand, may compromise identification of gastric or other organ pathology.
Endoscopy
Endoscopic evaluation and biopsy of the stomach is a commonly used procedure to evaluate the gastric mucosa in cats and dogs.12 Endoscopy permits direct observation of the lumen and provides a noninvasive method of obtaining biopsy specimens for histopathologic evaluation. Brush cytology, fluid aspirations with culture, and foreign-body retrieval are all possible during routine endoscopy.
Endoscopy provides direct observation of the lumen and mucosa, but may miss extramural diseases. Endoscopy has the additional disadvantages of equipment expense, the need for general anaesthesia, and the inability to obtain full-thickness biopsies. Furthermore, the procedure does not permit the diagnosis of motility disorder or partial obstructions of the intestine. Endoscopy procedures are described in further details in Chapter 27.
Histopathologic Evaluation for Inflammation
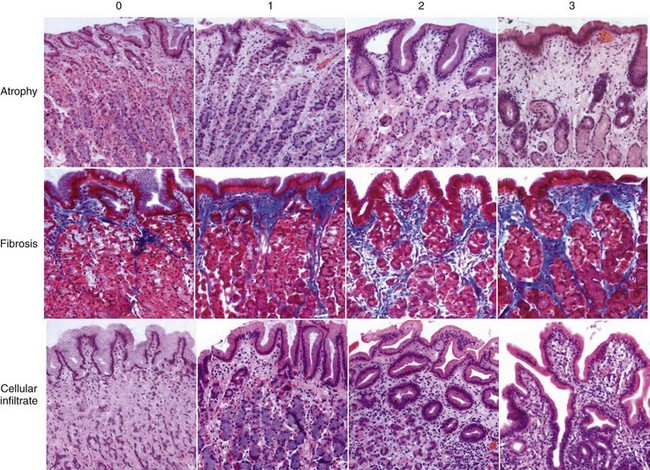
Gastritis is a common finding in chronic vomiting dogs and cats. A diagnosis of gastritis requires histopathologic examination of gastric biopsy specimens. The histopathologic evaluation of the biopsy specimens includes cell types and number of infiltrating cells (lymphocytes, plasma cells, mononuclear cells, eosinophils, and neutrophils) and the presence of atrophy or hypertrophy, metaplasia, fibrosis, edema, or lymphoid follicles. Disagreements do exist among classification systems used by different pathologists when evaluating gastric biopsy specimens, making it important for the clinician, together with the pathologist, to define the criteria upon which the diagnosis is made. Figure 56-7 illustrates a visual scale grading system from 0 to 3 based on a photographic scale of cellular infiltrate, atrophy, and fibroses. Grade 0 is normal gastric mucosa and grade 3 is severe gastritis.13 A standardized system of characterizing gastric cellularity and morphology has been developed by the World Small Animal Veterinary Association Gastrointestinal Standardization Group.14
There is an increasing interest in characterizing mucosal proinflammatory and immunomodulatory cytokines in cats and dogs with gastritis. Perhaps this will help the clinician’s understanding of the immunologic response in the stomach and help the clinician’s understanding of the mechanisms of gastritis.13 Gastric biopsies can also reveal infections with parasites (O. tricuspis) or bacteria (Helicobacter spp.).13,15,16 The reader is referred to other sections (Gastric Inflammation, Gastric Infection) in this chapter for more specific detail.
Evaluation for Gastric Helicobacter spp. Infection
Gastritis is often of unknown etiology in dogs and cats. H. pylori is accepted as an important pathogen in human gastroenterology and is believed to be the primary cause of chronic gastritis, gastric and duodenal ulceration, and even gastric carcinoma. For several years, interest in Helicobacter spp. infections as a cause of chronic gastritis in cats and dogs has been increasing. Several studies show that gastric infection with Helicobacter spp. is common in dogs, with a prevalence ranging from 67% to 100% in clinically healthy pet dogs, and 41% to 86% prevalence and in clinically healthy cats. Cats and dogs are infected with Helicobacter felis, Helicobacter bizzozeronii, Helicobacter heilmannii, Helicobacter salomonis, Helicobacter bilis, and Flexispira rappini.17–22 The dilemma is that the pathogenicity of Helicobacter spp. is well described in several other species, such as ferrets, mice, and cheetahs, but is still evolving in cats and dogs. Investigations of the pathogenicity of Helicobacter spp. in dogs and cats with naturally acquired infections have so far been inclusive and indicate an overlap in the type and severity of gastric inflammation in infected and uninfected dogs and cats.
Several methods are available to diagnose Helicobacter infection in cats and dogs. Mucosal biopsies and histopathologic evaluation have been used for many years. Helicobacter spp. can be recognized in a standard hematoxylin and eosin stain in the mucus and/or intracellularly in the epithelium. If Helicobacter spp. are present in very small numbers a Warthin-Starry silver stain may prove more useful.13,15 Helicobacter spp. are a strong producer of urease and mucosal biopsies can be tested for urease activity with an “on site” rapid urease tests (e.g., CLO [Campylobacter-like organism] test). Urease breaks down urea and produces a high local concentration of ammonia, which makes it possible for Helicobacter spp. to survive the low pH of the gastric lumen. Rapid urease tests consist of a urea-rich medium with a pH sensitivity dye. Recent use of antibiotic, bismuth, or acid secretory inhibitors in the patient may interfere with the test and provide false-negative results. The 13C breath test can be used to evaluate the effect of eradication.23,24
Polymerase chain reaction (PCR) techniques have proven very useful in the detection of Helicobacter spp. infections after DNA extraction of gastric biopsy specimens.13 This method includes use of genus and species-specific primers to detect species verification. Brush cytology of the mucus are useful for detecting Helicobacter spp. in both cats and dogs.25 The role of Helicobacter spp. in canine and feline gastritis is still unknown and much remains to be learned about the infection.
Exploratory Laparotomy
In some cases, exploratory laparotomy may be necessary to diagnose mural diseases of the GI tract that are beyond the reach of the endoscope, when perforation is suspected, or when multiorgan biopsy or full-thickness biopsy is required to obtain a definitive diagnose. At the time of exploratory laparotomy, full-thickness biopsy specimens of the stomach and intestine should always be considered, even with apparently normal macroscopic appearance. All abdominal organs must be examined and additional biopsies of the liver, lymph nodes, and masses should be obtained when indicated. If serum protein levels are low, this may influence tissue repair and increase the risk of biopsy-related complications. Fine-needle aspirates can be obtained from masses and lymph nodes when indicated. Cytology results may provide a preliminary result that will help the clinician initiate therapy before histopathology results are available. The complication rate following laparotomy is approximately 30% with 60% of the complications related to the primary disease and the rest related to surgical or anesthetic problems.26
Inflammation
Gastric disease is typically the result of inflammation, ulceration, neoplasia, or obstruction. Clinical manifestations include vomiting, hematemesis, melena, retching, belching, hypersalivation, abdominal distention, abdominal pain, and weight loss. The clinical approach is guided by considering gastric disease as a group of clinical syndromes that segregate on the basis of etiology, pathology, and clinical presentation (Table 56-3).1
Table 56-3 Diseases of the Stomach
| Clinical Syndrome | Predominant Features |
|---|---|
| Acute gastritis | Sudden onset of vomiting |
| Ulceration or erosion | Vomiting, hematemesis, melena, ± anemia |
| Gastric dilation volvulus | Nonproductive retching, abdominal distention, tachycardia |
| Chronic gastritis | Chronic vomiting of food or bile |
| Delayed gastric emptying | Acute to chronic vomiting more than 8 to 10 hours after feeding |
| Neoplasia | Chronic vomiting, weight loss, ± anemia |
Acute Gastritis
Etiology
Acute gastritis is the term applied to the syndrome of vomiting of sudden onset suspected to be associated with gastric mucosal insult or inflammation (Box 56-2). In most patients the cause is inferred from the history (e.g., dietary indiscretion), the diagnosis is rarely confirmed by biopsy, and treatment is more symptomatic and supportive than disease specific. Animals with acute gastritis associated with drug toxicity, foreign-body ingestion, or metabolic disorders frequently present with hematemesis, melena, concurrent diarrhea, or other signs of systemic illness and require a more thorough diagnostic approach to determine the cause and to provide optimal care. There is little evidence in the literature to suggest that viral infections such as parvovirus, distemper, or infectious canine hepatitis, have a role in acute gastritis.
Box 56-2
Causes of Acute Gastritis
• Dietary indiscretion or intolerance (nonallergic and allergic)
• Foreign bodies (e.g., bones, toys, hairballs)
• Drugs and toxins (e.g., antibiotics, digoxin, nonsteroidal antiinflammatory drugs, corticosteroids, heavy metals, plants, cleaners, bleach, dietary contaminants)
• Systemic disease (e.g., hypoadrenocorticism, uremia, liver disease)
• Parasites (e.g., Ollulanus, Physaloptera spp.)
• Bacteria (e.g., bacterial toxins, Helicobacter)
Treatment
Therapy for uncomplicated acute gastritis is an empirical combination of symptomatic and supportive agents such as fluids, dietary restriction and modification, mucosal protectants or adsorbents, and possibly antacids.2,3
Fluid Therapy
Small amounts of oral fluids, little and often, can be given in the face of vomiting, with the volume increasing and frequency decreasing as vomiting subsides. Subcutaneous administration of an isotonic balanced electrolyte solution may be sufficient to correct mild fluid deficits (<5%), but is insufficient for patients with moderate to severe dehydration. Patients requiring intravenous fluids should undergo a more extensive diagnostic evaluation. Chapter 48 has more detailed information on fluid therapy.
Dietary Restriction and Modification
Where vomiting is acute, oral intake is typically discontinued for 24 hours. However, a liquid diet can be offered in the face of vomiting to maintain GI barrier function.4 This can be transitioned to a homemade, nonspicy, fat-restricted, bland diet (e.g., boiled chicken and rice, low-fat cottage cheese and rice [1 : 3]) or a commercial fat-restricted, rice-based diet that is fed little and often. After a week or so, the normal diet can be reintroduced gradually.
Chronic Gastritis
Etiology
The diagnosis of chronic gastritis is currently based on the histologic examination of gastric biopsies and is subclassified according to histopathologic changes and etiology. Histopathologic evidence of gastritis is a common finding in dogs, with 35% of dogs investigated for chronic vomiting and 26% to 48% of asymptomatic dogs affected.5,6 The prevalence in cats has not been determined.
Gastritis in dogs and cats is usually categorized according to the nature of the predominant cellular infiltrate (e.g., eosinophilic, lymphocytic, plasmacytic, granulomatous, lymphoid follicular), the presence of architectural abnormalities (e.g., atrophy, hypertrophy, fibrosis, edema, ulceration, metaplasia), and their subjective severity (e.g., mild, moderate, severe). A standardized visual grading scheme has been proposed by Happonen et al.6 and has been adapted for pathologists.7
It should be noted that even with standardized grading schemes there is often poor agreement between pathologists.7,8 Gastritis in dogs and cats is most commonly described as mild to moderate superficial lymphoplasmacytic gastritis, with or without concomitant lymphoid follicle hyperplasia. Eosinophilic, granulomatous, atrophic, and hyperplastic gastritis are less commonly reported.
Pathophysiology
Although the basis of the immunologic response in canine and feline gastritis is unknown, recent studies have shed light on the immunologic environment in the GI tract and reveal a complex interplay between the microflora, epithelium, immune effector cells such as lymphocytes and macrophages, and soluble mediators such as chemokines and cytokines.9–14 In health, this system avoids active inflammation by antigen exclusion and the induction of immune tolerance. The development of intestinal inflammation in mice lacking interleukin (IL)-10, transforming growth factor (TGF)-β, or IL-2 indicates the central importance of cytokines in damping-down mucosal inflammation. In many of these murine models, GI inflammation only develops in the presence of endogenous intestinal microflora, leading to the hypothesis that spontaneous mucosal inflammation may be the result of a loss of tolerance to this microflora. The role of these mechanisms in outbred species such as the dog and cat remains to be determined, but clearly loss of tolerance to bacterial or dietary antigens should be considered.
The epithelial cell is also emerging as a central coordinator in the inflammatory response. Epithelial cells are involved in sensing luminal constituents (e.g., bacteria) through pattern recognition receptors (also known as Toll-like receptors) and coordinating the inflammatory response. Gram-negative or pathogenic bacteria can induce proinflammatory cytokine secretion (e.g., IL-8, IL-1β) by epithelial cells, whereas “commensals” or bacteria such as Streptococcus faecium or Lactobacillus spp. induce the production of the immunomodulatory cytokines TGF-β or IL-10.10 The proinflammatory cytokines produced by epithelial cells are modulated by the production of IL-10 from macrophages and potentially by the epithelial cells themselves.15 In this context, dogs with lymphoplasmacytic gastritis of undetermined etiology show a correlation between the expression of the immunomodulatory cytokine IL-10 and proinflammatory cytokines (interferon [IFN]-γ, IL-1β, IL-8).7 Mucosal pathology is related to cytokine messenger RNA (mRNA) expression (e.g., neutrophil infiltration in response to IL-8 and IFN-γ; macrophage and lymphocyte infiltration to IFN-γ; and fibrosis to IL-1β).
Histologic severity of lymphoplasmacytic gastritis in dogs correlates with atrophy, infiltration with lymphocytes and macrophages, and expression of IL-10 and IFN-γ.7 Simultaneous expression of IL-10 and IFN-γ mRNA has also been observed in the intestines of Beagle dogs (lamina propria cells and the intestinal epithelium) in the face of a luminal bacterial flora that was more numerous than that of control dogs.16 Thus it is tempting to visualize a “homeostatic loop” consisting of proinflammatory stimuli and responses, countered by immunomodulation and repair, with an imbalance in either of these arms manifest as gastritis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree