Chapter 20 Polyuria and Polydipsia
Definition
Polyuria (PU) is defined as excessive urine production and is confirmed by demonstrating that daily urine production exceeds the upper limit of normal. In dogs, daily urine volume should normally not exceed approximately 50 mL/kg/day.1 Normal daily urine volume is lower in cats. Frequency of voiding is not incl uded in the definition of PU and may be normal or increased in patients with PU. It is essential that PU not be confused with pollakiuria, the frequent voiding of small quantities of urine.
Polydipsia (PD) is consumption of water in excess of the upper limit of normal daily intake. Normal water intake in dogs is usually less than 60 mL/kg/day with an upper limit of 100 mL/kg/day.1 Because water consumption is easier to measure, it is often used to confirm PD. However, environmental and behavioral factors (e.g., ambient temperature, activity of the animal) may markedly alter water consumption in dogs and should be considered in interpreting these measurements.2 Normal values for water intake are likely substantially less in cats, but a well-defined cutoff value has not been established.
Polyuric patients should have reduced urine specific gravity values; therefore, urine concentration is often used as a surrogate for confirming PU. Patients that persistently produce urine less concentrated than expected (i.e., <1.030 for dogs and <1.035 for cats) should be evaluated for a possible pathologic defect in urine-concentrating ability.3 In dogs, urine samples obtained in the morning immediately upon arising are typically the most concentrated and thus provide a more reliable estimate of urine concentrating ability.4 Urinalysis should be performed to determine urine concentration in any patient in which the owner reports increases in water intake or urination, regardless of whether the actual measured water intake exceeds the upper limit for PD. Patients with documented PD that have concentrated urine should be evaluated for nonurinary water losses.
Pathophysiology and Mechanism
General Mechanisms Affecting Water Intake and Urine Volume
In contrast, isoosmotic fluid loss (e.g., many gastrointestinal and urinary fluid losses) typically reduces effective circulating volume without altering plasma osmolality. This decline in effective circulating volume is termed volume depletion rather than dehydration. With volume depletion, both sodium and water retention are needed to correct the volume deficit. Sensors and response system for maintaining effective circulating volume are predominantly focused on modifying renal sodium handling; fluid volume is regulated because water is retained or excreted with sodium.5 Factors active in this process include the renin–angiotensin–aldosterone system, atrial natriuretic peptide (ANP), ADH, and activation of thirst.
Water Balance and Polyuria and Polydipsia
In the steady state, water intake must equal water output. Water intake includes ingested water, water obtained from food, and water generated endogenously through oxidation of carbohydrates, proteins, and fat. Water output is the sum of urine volume and “insensible losses,” which includes water contained in stool as well as evaporative losses from the skin and respiratory tract. These insensible losses typically account for approximately 20 to 25 mL/kg/day; however, they may increase with diarrhea, tachypnea, or increased ambient temperature.6 When insensible losses increase, water intake may increase without a coincident increase in urine volume.
Differential Diagnosis
Table 20-1 summarizes various recognized causes of PU and PD. Many of the causes of PU or PD present with other clinical signs or laboratory abnormalities, which readily facilitate their diagnosis (Table 20-2). In most instances, the cause for PU or PD can be determined from information gathered from history, physical examination, a complete blood count, serum chemistry profile, and urinalysis. The most common causes of PU and PD in dogs include renal disease, hyperadrenocorticism, and diabetes mellitus, whereas the most common causes of PU in cats include renal disease, hyperthyroidism, and diabetes mellitus. Gastrointestinal disease and gastrointestinal leiomyosarcoma have been linked to PU and PD.7–9 Figure 20-1 is an algorithmic approach to diagnosis.
Table 20-1 Ruleouts for Polyuria and Polydipsia in Dogs and Cats
| Ruleout for PU and PD | Additional Tests Supporting Diagnosis |
|---|---|
| Renal disease | Glomerular filtration rate (GFR) measurement (creatinine or iothalamate clearance), imaging (ultrasound, radiographs) |
| Pyelonephritis | Urine culture, renal imaging (ultrasound, excretory urography), pyelocentesis (culture, cytology) |
| Leptospirosis | Serology and/or polymerase chain reaction (PCR) |
| Chronic partial urinary obstruction | Urinary tract imaging (ultrasound, contrast radiography) |
| Renal glucosuria or Fanconi disease | Fractional urinary excretion studies (urine amino acids, bicarbonate, glucose, magnesium, phosphate, potassium, sodium) |
| Primary nephrogenic diabetes insipidus | Failure to respond to ADH (usually an exclusion diagnosis, typically a congenital disorder) |
| Postobstructive diuresis | Evidence of recent urinary obstruction, serial declines in serum creatinine and urea nitrogen concentrations |
| Renal medullary solute washout | Seek underlying cause, response to gradual partial water deprivation |
| Diabetes mellitus | Serum fructosamine concentration |
| Hyperadrenocorticism | Adrenocorticotropic hormone (ACTH) response test or low-dose dexamethasone suppression test |
| Hyperthyroidism (cats) | Free thyroxine (T4) by equilibrium dialysis, thyroid scan |
| Hypoadrenocorticism | ACTH response test |
| Primary hyperaldosteronism | Plasma aldosterone, plasma aldosterone-to-renin ratio, oral fludrocortisone suppression test (urinary aldosterone-to-creatinine ratio) |
| Central diabetes insipidus | ADH response test, water-deprivation test, imaging (magnetic resonance imaging [MRI] or computed tomography [CT]) |
| Acromegaly | Feline plasma growth hormone concentration (± available), insulin-like growth factor-I concentrations, MRI or CT of the pituitary fossa |
| Pheochromocytoma | Blood pressure, abdominal ultrasound, urine catecholamine concentrations |
| Hypercalcemia Hypokalemia (marked) Hyponatremia (marked) | Blood ionized calcium concentration |
| Hepatic failure | Fasting and postprandial serum bile acids, imaging (ultrasound, radiographs) |
| Portosystemic shunt | Fasting and postprandial serum bile acids, imaging (abdominal ultrasound, portography, rectal scintigraphy) |
| Leiomyosarcoma | Imaging, endoscopy, biopsy |
| Gastrointestinal disease | Imaging, endoscopy, biopsy |
| Pyometra Polycythemia | Imaging (ultrasound, radiographs) |
| Pericardial effusion | Echocardiogram |
| Psychogenic (primary) PD | Multiple urine specific gravity determinations, ADH response test, water-deprivation testing |
| Paraneoplastic | Imaging (radiographs, ultrasound, CT or MR) |
| Sudden acquired retinal degeneration syndrome (SARDS) Iatrogenic | Retinal examination, electroretinogram |
ACTH, Adrenocorticotropic hormone; ADH, anti-diuretic hormone; CT, computerized tomography; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PD, polydipsia; PU, polyuria; SARDS, sudden acquired retinal degeneration syndrome; T4, thyroxine.
Table 20-2 Medical History and Physical Examination Findings Providing Clues to Possible Causes for Polyuria and Polydipsia
| Clinical Finding | Possible Ruleouts |
|---|---|
| Weight loss | Kidney disease, diabetes mellitus, hyperthyroidism, pyelonephritis, malignancy-induced hypercalcemia, hypoadrenocorticism, hepatic disease, pyometra |
| Polyphagia | Diabetes mellitus, hyperthyroidism, hyperadrenocorticism, acromegaly |
| Decreased appetite | Kidney disease, pyelonephritis, malignancy-induced hypercalcemia, hepatic disease, hypoadrenocorticism |
| Vomiting | Kidney disease, hypoadrenocorticism, pyelonephritis, hepatic failure, hypercalcemia, hypokalemia, hyperthyroidism, diabetes mellitus, consumption of excess water |
| Malaise and/or weakness | Kidney disease, hypoadrenocorticism, pyometra, hypercalcemia, diabetes mellitus, hepatic disease, hypokalemia, hyperadrenocorticism |
| Behavioral or central nervous system (CNS) signs (seizures, ataxia, stupor, blindness) | Hepatic failure, primary PD, central diabetes insipidus, hyperadrenocorticism, acromegaly, sudden acquired retinal degeneration syndrome (SARDS) |
| Marked PU or PD | Primary PD, central diabetes insipidus, congenital nephrogenic diabetes insipidus |
| Middle-aged female, recent estrus | Pyometra |
| Bilateral alopecia, skin disease | Hyperadrenocorticism or other endocrinologic disorders |
| Abdominal distention | Hepatic failure, hyperadrenocorticism, pyometra, nephrotic syndrome, bladder enlargement caused by PU |
| Hepatomegaly | Hyperadrenocorticism, diabetes mellitus, hepatic disease |
| Small kidneys | Chronic or congenital kidney disease |
| Hypertension, hypertensive retinopathy | Chronic kidney disease (CKD), hyperthyroidism, diabetes mellitus, hyperadrenocorticism |
| Uremic breath, uremic stomatitis | Kidney disease |
| Thyroid or neck mass | Hyperthyroidism, hyperparathyroidism, CKD |
| Heart murmur | Hyperthyroidism, CKD, acromegaly |
| Panting, tachypnea | Hyperadrenocorticism, hyperthyroidism, mediastinal mass (lymphoma), pheochromocytoma |
CKD, Chronic kidney disease; CNS, central nervous system; PD, polydipsia; PU, polyuria; SARDS, sudden acquired retinal degeneration syndrome.
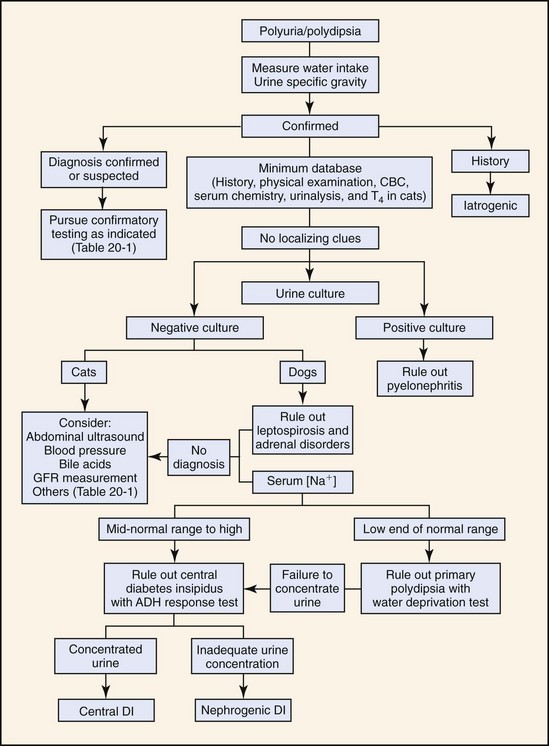
Figure 20-1 Algorithm demonstrating the diagnostic sequence recommended for establishing the diagnosis of PU and/or PD in dogs and cats. Tables 20-1 and 20-2 provide supplemental information useful in applying the algorithm to patients. ADH, Anti-diuretic hormone; CBC, complete blood count; DI, diabetes insipidus; GFR, glomerular filtration rate; T4, thyroxine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


