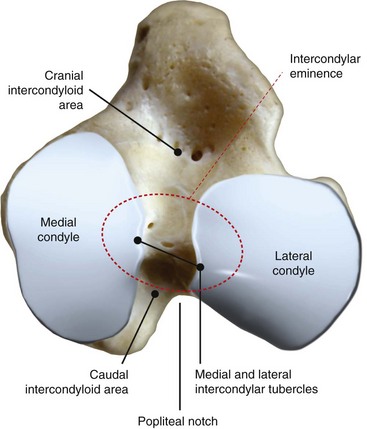
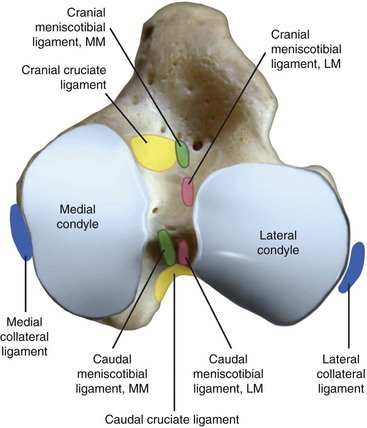
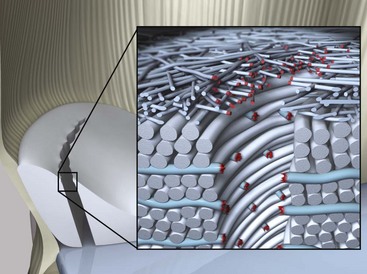
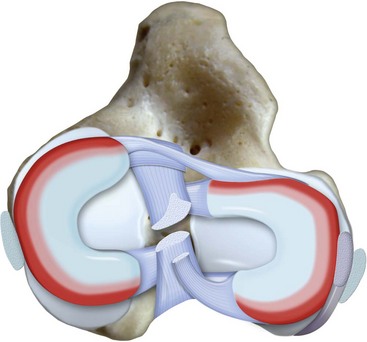
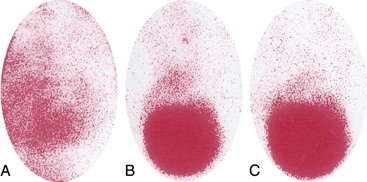
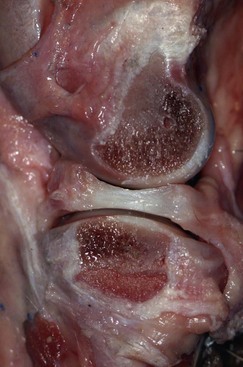
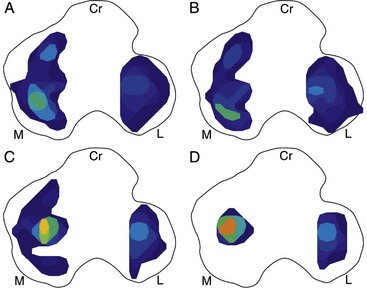
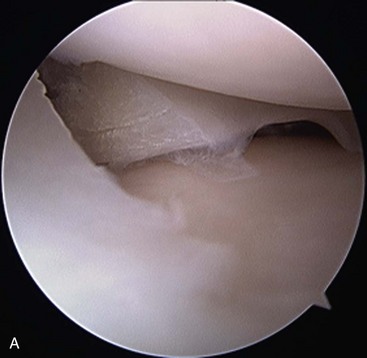
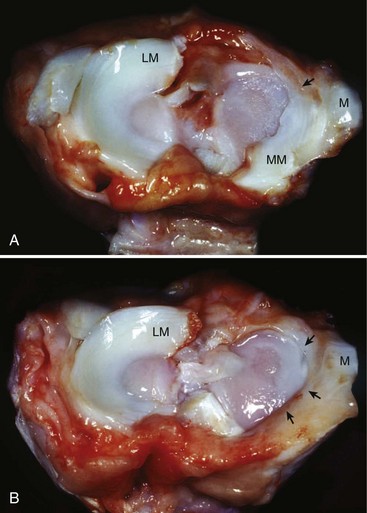
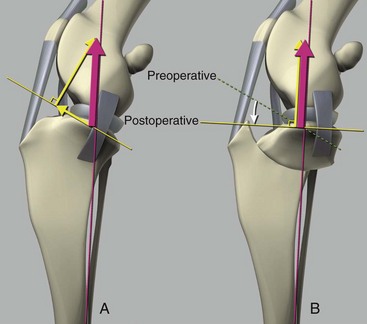
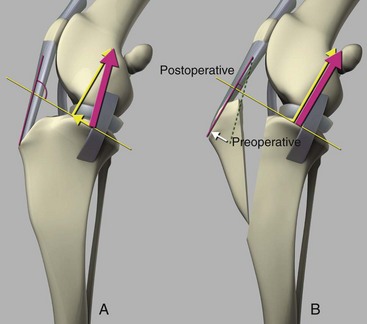
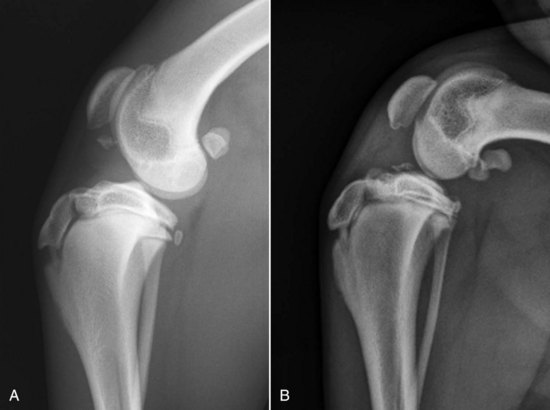
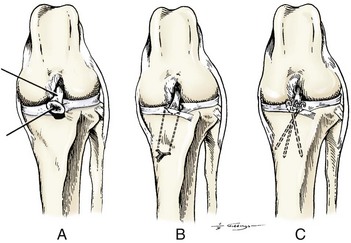
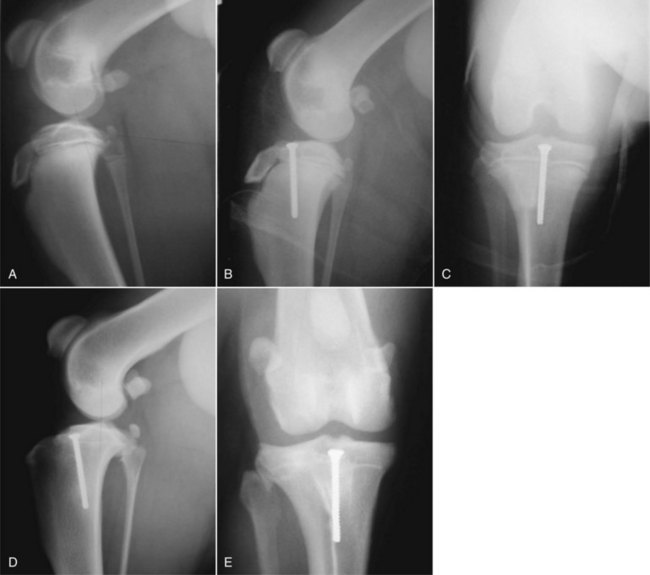
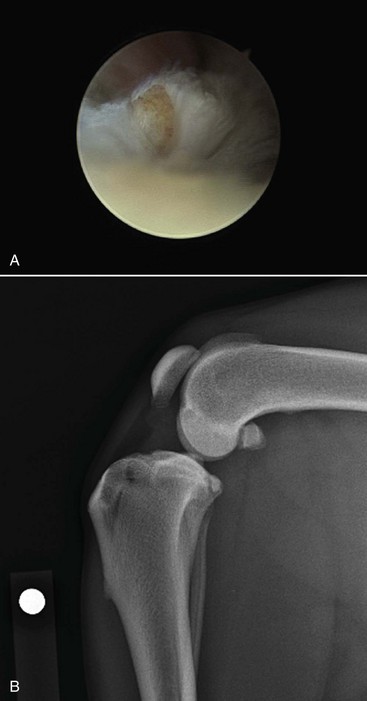
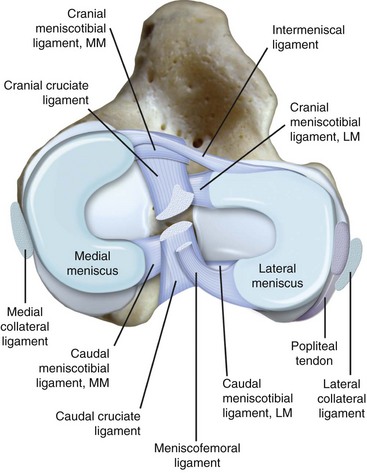
Chapter 62 The proximal end of the tibia is slightly convex in the sagittal plane and triangular in cross-section, with its apex cranial. The proximal articular surface is divided into medial and lateral condyles, and each has approximately the same surface area; however, the medial condyle is oval and the lateral is nearly circular. A sagittal, nonarticular area known as the intercondylar eminence separates these two articular areas. The medial and lateral intercondylar tubercles are triangular projections atop the intercondylar eminence; these tubercles articulate with the femur on their abaxial surfaces. The cranial intercondyloid area is an oval depression cranial to the intercondylar eminence that serves as the attachment site for the cranial cruciate ligament and the cranial meniscal ligaments (Figure 62-1). The caudal intercondyloid area is a smaller depression caudal to the intercondylar eminence that serves as an attachment site for the caudal meniscal ligaments. At the caudal-most aspect, the broad popliteal notch separates the tibial condyles; the caudal cruciate ligament attaches to the lateral edge of the popliteal notch. The extensor (muscular) groove is a small notch at the cranial margin of the lateral tibial condyle that extends to the margin of the articular surface of the lateral condyle. The long digital extensor tendon runs through this groove. In humans, the angular, cranial margin of the extensor groove is known as the tubercle of Gerdy.39 The tibial tuberosity is the large, quadrangular cranioproximal process that serves as the attachment for the patellar ligament, as well as for parts of the biceps femoris and sartorius muscles. The cranial border of the tibia (formerly called the tibial crest) extends distally from the tibial tuberosity. The obliquely placed facet for articulation of the fibular head, the facies articularis fibularis, is on the caudolateral surface of the tibial condyle. A small tubercle on the medial aspect of the head of the fibula articulates with the tibia at this facet. The menisci are attached to the tibia by a series of paired ligaments. The cranial meniscotibial ligament of the medial meniscus runs from the cranial axial border of the medial meniscus to attach to the tibia at the cranial intercondyloid area immediately cranial to the intermeniscal ligament, the attachment of the cranial meniscotibial ligament of the lateral meniscus, and the tibial attachment of the cranial cruciate ligament (Figure 62-2). The cranial meniscotibial ligament of the lateral meniscus attaches to the cranial intercondyloid area of the tibia just caudal to the attachment of the cranial meniscotibial ligament of the medial meniscus and the intermeniscal ligament (see Figure 62-2). The caudal meniscotibial ligament of the medial meniscus runs from the caudal axial border of the medial meniscus to attach to the caudal intercondyloid area of the tibia (see Figure 62-1). The caudal meniscotibial ligament of the lateral meniscus runs from the caudal axial border of the lateral meniscus to attach in the popliteal notch, just caudal to the caudal intercondyloid area (see Figure 62-2). These ligaments are also known as the cranial and caudal tibial ligaments of the respective menisci.118 The meniscofemoral ligament of the lateral meniscus, also known as the femoral ligament,118 runs dorsally from the caudal axial border of the lateral meniscus to attach within the intercondylar fossa of the femur as it blends into the medial femoral condyle. The intermeniscal ligament is a transverse fibrous band that extends from the caudal side of the cranial tibial ligament of the medial meniscus to the cranial side of the cranial tibial ligament of the lateral meniscus (see Figure 62-2).118 Figure 62-2 Menisci and ligaments of the stifle joint, dorsal aspect. MM, Medial meniscus; LM, lateral meniscus. Four femorotibial ligaments in the stifle joint provide primary ligamentous support: two collateral ligaments and two cruciate ligaments. The cruciate ligaments are located within the stifle joint (intra-articular), but because they are covered by synovium, they are considered to be extrasynovial. These ligaments are designated cranial and caudal based on their tibial attachment. In addition, they decussate, or cross, each other—hence their name. The cranial (lateral) cruciate ligament attaches to the caudomedial aspect of the lateral femoral condyle and the caudolateral part of the intercondyloid fossa of the femur and runs diagonally in a cranial, medial, and distal direction across the intercondyloid fossa to attach to the cranial intercondyloid area of the tibia.20,99,156 This ligament is grossly divided into a larger caudolateral part that attaches at the caudolateral aspect of the tibial attachment site and a smaller craniomedial band that attaches at the craniomedial aspect of the tibial attachment site. The craniomedial fibers spiral outward axially approximately 90 degrees as the ligament courses from proximal to distal.20 The caudal (medial) cruciate ligament attaches to the lateral surface of the medial femoral condyle and runs caudodistally to attach to the medial edge of the popliteal notch of the tibia, medial to the caudal meniscotibial ligament of the lateral meniscus. The caudolateral fibers spiral inward abaxially about 90 degrees as the ligament courses from proximal to distal.20 The caudal cruciate ligament is larger and longer than the cranial cruciate ligament. The cruciate ligaments comprise a core region of fascicles containing collagen fibrils and fibroblasts, and are covered by an epiligamentous region composed of synovial intima and underlying loose connective tissue. The epiligamentous region is absent only where the cranial cruciate ligament wraps around the caudal cruciate ligament.152 Abundant mechanoreceptors and proprioreceptors are located within the center of the ligament; innervation arises from afferent axons that penetrate from the synovium that envelops the ligament.18,388 The lateral (fibular) collateral ligament attaches proximally to an oval area on the lateral epicondyle of the femur and passes superficial to the tendon of origin of the popliteus muscle. The distal attachment is found primarily on the head of the fibula, with a few fibers attaching to the adjacent lateral condyle of the tibia.118,361 The medial (tibial) collateral ligament attaches proximally to an oval area on the medial epicondyle of the femur.361 As it courses distally, it blends with the joint capsule and has a strong attachment to the medial meniscus.361 It passes superficial to the tibial insertion of the semimembranosus muscle and over a bursa at the medial tibial condyle to attach distally to a long rectangular area at the medial border of the tibial metaphysis.361 The medial collateral ligament is fused with the joint capsule and the medial meniscus, while the lateral collateral ligament is attached only loosely to the joint capsule and is separated from the lateral meniscus by the tendon of origin of the popliteus muscle.361 The thin medial and lateral femoropatellar ligaments are continuations of the femoral fascia that originate from the sides of the patella. The lateral femoropatellar ligament is better defined and attaches to the lateral fabella. The poorly defined medial femoropatellar ligament blends with the periosteum at the medial epicondyle of the femur. Shape, Attachment, and Function: The menisci are C-shaped disks of fibrocartilage located between the condyles of the femur and the tibia. The crescent shape and the roughly triangular cross-section of the menisci adapt to the femoral and tibial articulating surfaces and improve joint congruity. The peripheral border of each meniscus is thick, convex, and attached to the inside of the joint capsule; the axial border tapers to a thin free edge. The proximal surfaces of the menisci are concave and are in contact with the femoral condyles, whereas the distal surfaces of the menisci are flat and rest on the tibial condyles.72,118,221 The menisci are an example of a specific structure-function relationship. Their wedge shape and nearly frictionless surface cause radial extrusive forces to be developed by joint compressive forces. The radial force that develops when the joint is weight-loaded is resisted by the tensile stress developed in the circumferentially arranged collagen fibers of the tissue. This tensile stress is referred to as hoop stress. The menisci, a functional extension of the tibia, are held in place by ligaments and soft tissue attachments (see Figure 62-2). The cranial and caudal meniscal horns are firmly attached to bone via the ligaments (cranial and caudal meniscotibial ligaments of the menisci) described in the previous section. These ligaments are fundamental for the load distribution function of the menisci because they resist the hoop forces (see meniscal function section) that develop in the meniscus under axial load. The meniscal body is anchored less firmly to the tibia and femur through the coronary ligament, which varies between medial and lateral menisci.72,118,221 The medial meniscus is firmly attached to the medial collateral ligament and the joint capsule via the coronary ligament, but the lateral meniscus lacks these attachments. 72 Familiarity with normal meniscal attachment patterns is critical for understanding the mechanism of meniscal rupture and for performing surgical procedures on the meniscus. The medial meniscus is firmly attached to the tibia through the cranial and caudal meniscotibial ligaments (see Figure 62-2). The intermeniscal ligament is located between the cranial horns of the lateral and medial menisci. The significance of this ligament is not well known, but it likely contributes to stability of the cranial horns and hoop tension. An understanding of the location of this ligament is necessary for avoiding injury during surgical procedures performed in close proximity to the intermeniscal ligament. The caudal tibial ligament of the medial meniscus is a flat, fan-shaped structure that extends to the most caudal aspect of the medial tibial plateau. Its caudal location should be considered when meniscal procedures such as caudal meniscal release and caudal hemimeniscectomy are performed, because its most caudal aspect cannot be viewed via arthroscopy or arthrotomy. The periphery of the medial meniscus is attached to the joint capsule (and indirectly to the tibia and femur) and the medial collateral ligament through the coronary ligament that extends along most of the meniscus. The abaxial border of the medial meniscus blends into the medial collateral ligament, providing another strong attachment to the tibia.72,118,221 The lateral meniscus is less firmly attached to the tibia than is the medial meniscus. The cranial tibial ligament of the lateral meniscus anchors the cranial horn to the cranial intercondyloid area of the tibia cranial to the intercondylar eminence and caudal to the tibial attachment of the cranial cruciate ligament (Figure 62-3; see also Figures 62-1 and 62-2). Small caudal tibial ligament(s) of the lateral meniscus may or may not be present in dogs and can be attached cranial or caudal to the tibial attachment of the caudal cruciate ligament, and on the lateral aspect of the popliteal notch when present.143,221 These ligaments may consist of a thin and nonfibrous connection between the caudal horn and the tibia.145 The caudal horn of the lateral meniscus is also firmly attached to the intercondyloid fossa of the femur as it blends into the medial surface of the medial femoral condyle by the meniscofemoral ligament. The lateral meniscus has less extensive attachment to the joint capsule than does the medial meniscus because the popliteus muscle tendon is interposed between the joint capsule and the lateral meniscus. Additionally, because of the lack of an attachment to the lateral collateral ligament, the abaxial aspect of the lateral meniscus forms the margin of a groove within which the popliteal tendon can glide (see Figure 62-2). Popliteal-meniscal fascicles attach the lateral meniscus to the popliteal tendon along the lateral and caudal aspect of the groove.143 The anchorage of the lateral meniscus to the femur and its close relationship with the popliteal tendon couple its motion with that of the femoral condyle during rotation. It is therefore less likely to be injured than the relatively immobile medial meniscus.143 Composition: The menisci are fibrocartilaginous structures that are primarily composed of an interlacing network of collagen fibers (predominantly type I collagen) interposed with cells and an extracellular matrix of proteoglycans and glycoproteins.186,333 The collagen fibrils within this matrix are highly structured into three layers that together allow compressive forces to be dissipated both peripherally and tangentially into hoop stresses. This distribution creates an extremely effective mechanism of load sharing among the body and horns of the meniscus. The collagen bundles of the surface layer are randomly oriented with a compositional similarity to articular hyaline cartilage. This relates to its function, which is to allow low-friction motion between the meniscal tissue and the femur and tibia as the femoral condyles slide against the menisci and move over the tibial plateau during knee motion.60 In the deeper layers of the meniscal tissue are two structurally distinct regions where the orientation of collagen fibers is different: in the innermost third, the collagen bundles predominantly lie in a radial pattern, whereas in the outer two thirds, the collagen bundles are orientated circumferentially (Figure 62-4).60,186,231 This suggests that the inner third may function in compression, and that the outer two thirds may function in tension. Observed less frequently are radially oriented collagen fibers within the bulk of the meniscal tissue; it is thought that they may act as tie fibers, resisting longitudinal splitting of circumferential collagen bundles.60,231 Other important components of the extracellular matrix of the meniscus are the proteoglycans, which are large negatively charged hydrophilic molecules that can entrap water 50 times their weight in free solution.2 The ability of the proteoglycans to form aggregates helps to immobilize them within the collagen fibrillar meshwork. Proteoglycans provide the tissue with a high capacity to resist large compressive loads and play an important role in determining the material properties of the meniscus.126 Based on the biphasic theory developed by Mow et al.,259 the meniscus is a biphasic material with a solid matrix phase and an interstitial fluid phase. Thus, when a load is applied to meniscal tissue, the solid phase, consisting predominantly of circumferentially oriented collagen bundles, shows an elastic response. However, simultaneously, the compressive load is carried by the fluid as it is very slowly extruded at a rate dependent on the permeability of the tissue and the viscosity of the fluid.229 This behavior is also time dependent; thus with dynamic compression at high loading frequencies, the menisci behave as a rubber-like elastic material, but at lower frequencies, significant viscous dissipation occurs.64 The magnitude of both static and dynamic compressive moduli depends mostly on the extracellular matrix composition, as they increase with increasing glycosaminoglycan content and decrease with increasing water content.64 The close relationship of structure and function within the meniscus suggests that pathologic changes causing biochemical alterations in the meniscal matrix also affect biomechanical properties.263 Blood Supply: The blood supply of the canine meniscus originates from a small reflection of the vascular layer of the synovium, the synovial fringe, present on the femoral and tibial surfaces of the meniscus.25 These blood vessels supply the peripheral 15% to 25% of the menisci. This region is called the red-red zone because of the rich blood supply (Figure 62-5). The rest of the meniscus is mostly avascular and is divided into the axial white-white zone and an intermediate zone called red-white, with only a small number of vessels reaching this latter zone. The blood supply is organized in a perimeniscal capillary plexus, which originates from the medial and lateral genicular arteries. The relative avascularity of the meniscus plays an important role in decision making on meniscal treatment. The peripheral blood supply to the meniscus provides a rationale for repair of tears in the red-red zone.25 Other factors such as the presence of degeneration and the chronicity of the injury should also be considered when one is deciding whether to repair or resect a damaged meniscus. The primary motion of the stifle joint consists of flexion and extension, and this occurs in the sagittal plane. Because of constraints imposed by the collateral ligaments, the cruciate ligaments, the menisci, and the cam-shaped geometry of the femoral condyles, strict uniplanar motion does not occur.26,113,233 The secondary type of motion consists of rotary movement of the tibia on the femur; this occurs in the transverse plane. The normal range of motion is approximately 140 degrees225 in flexion and extension. Median flexion and extension angles measured by goniometry in Labrador Retrievers have been reported as 41 degrees and 162 degrees, respectively, yielding a range of motion of 121 degrees.177 In extension, the medial and lateral collateral ligaments are taut, and they serve as primary stabilizers that limit internal and external rotation of the tibia. In flexion, the femoral and tibial attachments of the lateral collateral ligament move closer together, resulting in laxity; in contrast, most of the medial collateral ligament remains taut, except for the caudal border, which shows some loosening.361 Relaxation of the lateral collateral ligament as the joint is flexed allows the lateral femoral condyle to displace caudally on the lateral tibial condyle, resulting in internal rotation of the tibia.361 Conversely, as the joint is extended, the lateral collateral ligament tightens, drawing the lateral femoral condyle cranially with respect to the lateral tibial condyle, resulting in external rotation of the tibia.361 In humans, the sequential axial rotation in the normal knee joint has been classically described as the “screw home” mechanism.26 Because the femoral condyles are cam shaped and are not spherical, a small amount of craniocaudal translation of the tibia with respect to the femur also occurs in the sagittal plane during flexion and extension. The femoral condyles roll caudally with flexion and cranially with extension with respect to the tibial condyles.23 The stifle joint is capable of slight varus (medial) and valgus (lateral) angulation in the frontal plane. In full extension, the medial collateral ligament limits valgus angulation, and varus angulation is limited by the lateral collateral ligament and the cranial cruciate ligament.247 At 90 degrees of flexion, all four femorotibial ligaments limit valgus angulation, while the lateral collateral, cranial cruciate, and caudal cruciate ligaments limit varus angulation.247 In addition to bony restraints and ligamentous constraints, excessive joint motion is prevented by a complex system of reflex arcs that involve modulation of the major muscle groups about the stifle329 by a series of mechanoreceptors and proprioreceptors.18,388 Joint loading that causes increased strain in the cranial cruciate ligament results in simultaneous contraction of the caudal thigh muscles and relaxation of the quadriceps femoris muscle.329 This is a protective feedback mechanism because in human beings, it has been shown that contraction of the quadriceps femoris muscle increases cranial cruciate ligament strain, but hamstring contraction reduces strain.242,329 It is interesting to note that a similar muscular synergism has been demonstrated in anterior cruciate ligament–deficient human beings, suggesting that an alternative reflex arc that is not dependent on anterior cruciate ligament receptors helps to maintain joint stability.329 The cranial cruciate ligament is the primary restraint against cranial tibial translation with respect to the femur (cranial drawer) and hyperextension.20,156 The cranial cruciate ligament and the caudal cruciate ligament twist on themselves to limit internal rotation, but neither ligament significantly limits external rotation.20 The cranial and caudal cruciate ligaments play variable roles in limiting varus and valgus angulation, as described previously. The grossly visible fascicles of the cranial cruciate ligament generally traverse the length of the ligament without crossing or interweaving,79 thus each is capable of sequential tightening and relaxation to maintain joint stability throughout a range of motion.374 Nevertheless, the twist in the cranial cruciate ligament results in the gross appearance of two fairly distinct and functionally discrete bands, especially in flexion.20 The craniomedial band is taut in both flexion and extension; therefore it is the primary check against cranial tibial subluxation.20 The caudolateral part is taut in extension and lax in flexion; thus it is the secondary check against cranial tibial subluxation.20 However, this gross functional division is likely an oversimplification, because it has been shown that the anterior cruciate ligament in human beings is functionally complex and consists of multiple portions that undergo changes in length and shifts in load distribution throughout the range of motion.337 The caudal cruciate ligament is the primary restraint against caudal tibial translation with respect to the femur (caudal drawer); it helps limit internal rotation of the tibia by twisting together with the cranial cruciate ligament.20,156 It comprises a larger cranial part that is taut in flexion and lax in extension, and a smaller caudal band that is lax in flexion and taut in extension.20 The caudal cruciate ligament is a secondary restraint against hyperextension20 and helps to limit varus and valgus angulation in flexion.247 The menisci provide several vital functions in the stifle joint, including load bearing, load distribution, shock absorption, and joint stability. Their wedge shape is ideal to act as a spacer between the femoral condyle and the tibial plateau, and when no compressive weight-bearing loads are present across the joint, it limits contact between articular surfaces. Under static-loading conditions, the menisci assume a significant load-bearing function in the stifle joint. As the joint is loaded, contact between the femoral condyle and the meniscus increases, and the larger contact area created by the meniscal-articular interface lowers the stress of the articular cartilage of the femur and tibia, protecting against mechanical damage to both the chondrocytes and extracellular matrix. Together, the menisci bear between 40% and 70% of the load across the stifle joint.201 The role of the meniscus as a weight-bearing structure was demonstrated in a cadaveric study that evaluated the effect of segmental hemimeniscectomy and meniscal release on contact pressures in the medial compartment of the stifle joint. Removal of the caudal horn of the medial meniscus causes a focal area of high pressure in the caudal region of the medial tibial condyle, corresponding to pressures higher than 10 MPa.286 This alteration of articular cartilage contact pressures is one of the factors contributing to articular cartilage degeneration following meniscectomy.88,119,387 The response of the menisci to loads applied to the stifle joint results from their macrogeometry, their well-organized structure, and the nature of their proximal and distal attachments.126 Different models demonstrate that the meniscus absorbs energy by undergoing elongation as a load is applied to the knee. As the joint compresses, the wedge-shaped meniscus extrudes peripherally, and its circumferentially oriented collagen fibers elongate (hoop stress). The force required to restrain the radial extrusion of the meniscus is derived from the large tensile hoop stress developed within the strong circumferential collagen fiber bundles caused by the extrusive effect. These hoop forces are transmitted to the tibia through the strong cranial and caudal attachments of the menisci. The importance of an intact functional unit (meniscal ligaments, meniscal body, and peripheral rim) is evident when the effect of meniscal release on load transmission across the joint is considered. Transection of the caudal meniscotibial ligament of the medial meniscus causes a 140% increase in peak contact pressure and a 50% decrease in contact area.284 A midbody (central) meniscal release and a hemimeniscectomy cause similar changes26 (Figure 62-6), further demonstrating that the meniscus should be considered as a whole functional unit that includes intact ligaments, the peripheral rim, and most of the meniscal body. The concept of the meniscus as a functional unit should serve as the basis for decision making in meniscal treatment. The menisci contribute to joint stability by increasing the congruity of the femorotibial joint.285 The menisci provide concavity to the relatively convex tibial plateau. Their effect is evident when it is considered that the meniscus functionally decreases the tibial plateau slope (Figure 62-7), as the prominent caudal horn effectively raises the caudal aspect of the tibial plateau. The role of the menisci is different between the cranial cruciate ligament–deficient stifle joint and the intact stifle joint.285 In the cranial cruciate ligament–deficient stifle joint, the caudal pole of the meniscus acts as a wedge, preventing the tibia from further subluxation and thereby playing a primary role in joint stability. This wedge effect increases the risk of meniscal tear in the untreated cranial cruciate ligament–deficient joint. In the normal stifle joint, loss of the meniscus causes minimal translation, suggesting a secondary role in joint stability of the meniscus when the cranial cruciate ligament is intact. The contribution of the meniscus to joint stability and kinematics in the cranial cruciate ligament–deficient stifle joint depends on the magnitude of instability. In a cadaveric study investigating the contribution of the meniscus to craniocaudal stability in cranial cruciate ligament–deficient stifles stabilized by tibial plateau leveling osteotomy, tibial plateau leveling osteotomy eliminated the wedge effect of the meniscus, suggesting a protective effect of tibial plateau leveling osteotomy against postliminary (occurring after the index [initial] surgical procedure) meniscal tears.285 However, as suggested by the incidence of postliminary meniscal tears,74,274,349 tibial plateau leveling osteotomy may be less likely to protect the meniscus during activities other than straight-line walking. In more provocative gaits, internal-external rotational instability may be more relevant than simple craniocaudal translation, as reported during walking on a treadmill.344 The biomechanics of meniscal treatments in dogs has been extensively studied in an attempt to understand its effect on meniscal and joint function.188,201,284–287 As noted through the work of Fairbank, total meniscectomy is not a benign operation.119 Cox and others found that meniscectomies in stifle joints lead to gross and microscopic degenerative changes in the articular cartilage. They noted that partial meniscectomies lead to less severe degenerative changes.88 They believed there was a direct relationship between the degree of degenerative change and the amount of meniscus removed. This supposition was recently confirmed in a cadaveric study evaluating the effect of serial meniscectomies on femorotibial contact pressures and on meniscal strain.287 The authors reported that smaller (30% radial width) partial meniscectomies had minimal effects on the biomechanics of meniscal function, whereas larger partial (75% radial width) and hemimeniscectomies resulted in significant changes in meniscal and femorotibial contact mechanics (Figure 62-8).287 The authors of this study speculated that loss of peripheral meniscal tissue eliminates the spacer effect of the meniscus, which is necessary for hoop tension to develop. The authors suggested that to act as an effective functional unit, the meniscus needs more than 25% of the radial width of the peripheral meniscal tissue. Using a similar ex vivo model, 75% partial meniscectomy was compared with meniscal repair to evaluate whether repairing a vertical longitudinal tear located in the red-red zone could be advantageous for the load-bearing function of the meniscus. Meniscal repair was effective in decreasing the peak contact pressure and increasing the contact area caused by a bucket-handle tear.348 In contrast, partial meniscectomy did not change the contact pressure pattern noted under the torn meniscus. Although clinical recommendations should not be based on cadaveric studies, interpretation of the large body of literature investigating the in vivo effect of meniscectomy on progression of osteoarthritis88,387 strongly indicates that a prudent approach to meniscal resection is to preserve the greatest amount of functional meniscal tissue. A conservative approach may also be valid for subclinical meniscal tears because a torn meniscus may continue to facilitate load transmission, as shown in the case of stable vertical longitudinal tears in a laboratory study.347 Meniscal release is complete transection of the medial meniscus at the meniscotibial ligament or through the midbody of the meniscus (Figure 62-9), with the goal of eliminating the wedge effect of the caudal horn of the medial meniscus during femorotibial subluxation. Meniscal release has been investigated in both in vivo and ex vivo experiments, to determine its biomechanical and biologic effects on joint function.188,222,284,286 Transection of the caudal meniscotibial ligament of the medial meniscus caused a 50% decrease in contact area and a 140% increase in the magnitude of pressure on the medial compartment of a cranial cruciate ligament–deficient stifle joint treated with a tibial plateau leveling osteotomy.284 In addition, a significant caudal shift of load transmission was noted after release. The abnormal contact mechanics following transection of the meniscotibial ligament was similar to that observed after a midbody meniscal release; based on these findings, a caudal release would not appear to confer a biomechanical advantage compared with a midbody meniscal release.284,286 As a result of these biomechanical alterations, supraphysiologic loading of articular cartilage may induce an upregulation in synthesis and degradation of cartilage matrix, which ultimately may lead to osteoarthritis. It is interesting to note that the focal high-pressure area observed in cadaveric experiments after meniscal release284,286 is consistent with the focal cartilage defect that Luther et al. reported in an in vivo experimental model of caudal release.222 It is likely that a combination of inflammatory and degradative mediators originating from the transected meniscus and biomechanical abnormalities from the loss of hoop tension play key roles in the development of cartilage degeneration following caudal release.222 Meniscal release is equivalent to caudal hemimeniscectomy with regard to meniscal function, further supporting the importance of an intact functional unit formed by the meniscal ligaments and the peripheral rim.287 The effects of medial caudal hemimeniscectomy and total meniscectomy were investigated in vivo in stifle joints of clinically normal dogs to evaluate their effect with respect to development of osteoarthritis (Figure 62-10).183 The results of this study indicate that the severity of secondary osteoarthritis induced in stifle joints at 16 weeks after both types of meniscectomies is similar in terms of pathologic changes in the joint, but the less radical meniscal excision is associated with less disruption of chondrocyte metabolism.183 Additionally, this study confirmed that incomplete meniscal regeneration, a semilunar-shape regrowth structure derived from the synovial membrane, is formed in 3 to 6 months.183 It is important to understand that this regenerated meniscus is not as functional as the original meniscus and does not prevent the development of osteoarthritis.101,183 It is likely that osteoarthritis of the cranial cruciate ligament–deficient stifle joint is initiated and possibly driven by abnormal dynamic joint function. Kinematic evaluation using surface markers is limited to flexion-extension measurement because estimation of three-dimensional bone movements from data collected from the skin surface is difficult.95 Despite this limitation, it has been demonstrated that the cranial cruciate ligament–deficient stifle joint remains more flexed throughout the gait cycle. The coxofemoral and tarsal joints respond to this increased flexion by remaining more extended during the stance phase than in the normal gait cycle.95 In addition to this kinematic alteration, kinetic analysis has revealed decreases in peak vertical forces and impulses, as well as braking and propulsion impulses.59,95 In one report, the peak vertical force on the normal hindlimb was 70% of the static body weight of the dog. Following cranial cruciate ligament transection, the peak vertical force was 25% of body weight at 2 weeks, 32% at 6 weeks, and 37% at 12 weeks.269 In a study utilizing an instrumented spatial linkage secured to bone plates, full, six degrees of freedom kinematic data were collected in five dogs. This study demonstrated a significantly increased cranial subluxation of the tibia (approximately 8 to 12 mm) with respect to the femur during the stance phase of the gait. In most animals, cranial tibial subluxation was unchanged during the swing phase. In one of the five dogs, cranial tibial subluxation persisted during the swing phase, and the authors speculated that this was due to failure of secondary restraints such as the menisci in this individual, resulting from chronic, cyclic cranial tibial subluxation and reduction.196 Dynamic radiostereometric analysis (RSA) utilizing dual digital video radiographic capture was used to serially assess stifle joint kinematics following cranial cruciate ligament transection in a cohort of 18 dogs, with a cohort of 5 dogs acting as sham controls.343 Following cranial cruciate ligament transection, peak cranial tibial translation increased by an average of 10 millimeters compared with the cranial cruciate ligament intact condition. Two months following cranial cruciate ligament transection, cranial tibial subluxation was evident only during stance phase and not during paw strike. However, 2 years following cranial cruciate ligament transection, an average of 5 millimeters of cranial tibial translation was present at the terminal swing phase.343 The authors suggested that the intact medial meniscus may serve as a “return spring” in the cranial cruciate–deficient stifle joint, elastically deforming during periods of cranial tibial subluxation, and then reducing tibial subluxation once stance phase load is removed343; also remove extra space (CCJ) this was substantiated by a cadaveric study in which the medial meniscus was found to be an important secondary stabilizer in the canine stifle joint.285 Further, it was suggested that long-term joint instability leading to joint capsule fibrosis and meniscal injury may cause a reduction in static joint laxity and elasticity over time.343 Significant changes in internal rotation of the tibia were not noted following cranial cruciate ligament transection; however, the range of abduction and adduction of the stifle joint was nearly doubled at 2 months postoperatively and remained significantly increased at 2 years postoperatively.343 Stifle joint flexion increased significantly until 6 months following transection. In addition, a significant increase in medial translation of the tibia was found following cranial cruciate ligament transection; this persisted until final follow-up 2 years after cranial cruciate ligament transection.343 In the dog, the dynamic nature of instability in the cranial cruciate ligament–deficient stifle joint was first clinically described by Henderson and Milton in 1978, when they described the tibial compression test as a diagnostic test for evaluation of the integrity of the cranial cruciate ligament.157 In this report, they recognized that two major factors account for the joint compressive force between the tibia and the femur: direct forces of weight bearing, and contraction of the gastrocnemius muscle.157 In 1984, Slocum described the cranial tibial thrust force as a shear force generated in the stifle during weight bearing that acts to thrust the tibia cranially.322 The authors concluded that the cranial tibial thrust force is a result of tibial compression and the slope of the tibial plateau.322 Slocum proposed the Active Model of the Stifle in 1993 (Figure 62-11).325 In this model, stifle joint stability is maintained by a synergism between the muscle forces responsible for stifle joint flexion and extension, the cranial tibial thrust force, the pull of the stifle flexor muscles of the thigh, and the passive restraints of the stifle joint including the cranial cruciate ligament and the caudal pole of the medial meniscus (Figure 62-11, A).325 Based on this model, the magnitude of the cranial tibial thrust force is dependent on the magnitude of the joint compressive force and the slope of the tibial plateau. In the normal stifle joint, the cranial tibial thrust force is counteracted by active elements such as the caudal thigh muscles and by passive elements including the cranial cruciate ligament and the caudal pole of the medial meniscus. According to this model, if the cranial tibial thrust force is not neutralized by the pull of the stifle flexors of the thigh, the cranial cruciate ligament begins to rupture or ruptures, and cranial tibial subluxation occurs during the stance phase of the gait. Leveling the tibial plateau (Figure 62-11, B) reduces the magnitude of the cranial tibial thrust force to regain stifle joint stability during the stance phase of the gait.322,325 An alternative biomechanical model of the stifle was proposed by Tepic in 2002 (Figure 62-12), based on the work of Nisell.262 The Tepic model suggests that the total joint force (joint reaction force) is not parallel to the functional axis of the tibia as proposed by Slocum, but instead is parallel to the patellar ligament (Figure 62-12, A). Tepic theorized that in Slocum’s model, which was developed based on analysis of the tibial compression test, the total joint force was presumed to be parallel to the Achilles tendon, which is essentially parallel to the functional axis of the tibia as defined by Slocum. However, in contrast to Slocum, it was theorized that under weight-bearing conditions, the force applied to the paw is not similar to the moment applied to the paw during the tibial compression test. Instead, Tepic suggests that the force applied to the paw is parallel to the patellar ligament. Thus, based on this model, stabilization procedures should be aimed at leveling the tibial plateau such that it is perpendicular to the patellar ligament, or altering the angle of the patellar ligament such that it is perpendicular to the tibial plateau (Figure 62-12, B).345 An alternative method of patellar tendon (ligament) angle (PTA) measurement based on the common tangent at the tibiofemoral contact point was a further refinement of this model.96 In skeletally immature animals, the attachment of ligament to bone by Sharpey’s fibers may be materially stronger than the bone itself. Thus, an acute overload of the ligament, such as a fall from a height, may result in avulsion of the ligament and a small piece of bone from the femoral (Figure 62-13, A) or, more commonly, the tibial (Figure 62-13, B) attachment site, rather than a midsubstance ligamentous tear. Because this injury more closely resembles an avulsion fracture, rather than ligamentous injury, it may be amenable to primary repair by reattachment of the avulsed fragment to bone (Figure 62-14). Proximal tibial epiphysiodesis (i.e., surgically induced premature union of the epiphysis with the diaphysis) has been described as a sole treatment to reduce the tibial plateau angle in young dogs with cranial cruciate ligament–deficient stifle joints, or to augment primary repair of a ligamentous avulsion (Figure 62-15, A).366 A Kirschner wire is inserted into the center of the cranial intercondyloid area of the tibia and is oriented parallel to the tibial shaft. Correct placement of the Kirschner wire is confirmed by intraoperative fluoroscopy or radiography, and a cannulated bone screw is advanced over the Kirschner wire (Figure 62-15, B–C). Epiphysiodesis occurs in the cranial aspect of the physis while the caudal aspect of the physis continues to grow, resulting in a reduction of the tibial plateau angle as long as residual growth remains at the time of surgery (Figure 62-15, D–E). In a case series of 22 joints, a reduction in the tibial plateau angle of 4 degrees to 24 degrees was observed, and an improved or normal gait was evident at follow-up examination in 18 of 22 joints.366 Valgus deformity occurred in 3 of 22 joints as the result of eccentric insertion and/or angulation of the screw (see Figure 62-14, C and E),underscoring the importance of precise screw positioning. The authors suggest intraoperative verification of Kirschner wire and screw position by fluoroscopy or radiography.366 Excessive limb loading, traumatic hyperextension, and/or excessive internal rotation of the tibia may overload the cranial cruciate ligament, resulting in acute rupture. This is a rare injury; however, when it occurs, dramatic pain, joint effusion, severe lameness, and stifle joint instability are present. This injury usually results in a midsubstance “mop end” tear (Figure 62-16, A), and radiographic findings include a dramatic effacement of the infrapatellar fat pad by a soft tissue opacity in the lateral view, consistent with edema of the fat pad and/or joint effusion, and the absence of osteophytosis (Figure 62-16, B). Despite years of clinical and basic science investigation since the description of cruciate ligament disease in the dog in 1926, the cause and pathogenesis of this extremely common disorder remain elusive. Biomechanical evaluations of intact cranial cruciate ligament preparations have demonstrated a decrease in material properties (modulus of elasticity, maximum stress, strain energy) with aging. These changes were more pronounced and occurred at an earlier age in dogs weighing more than 15 kg as compared with dogs weighing less than 15 kg.363 Histologic evaluation of these ligaments revealed evidence of degenerative changes, such as loss and metaplasia of ligamentocytes and failure to maintain collagen fibers.363 These findings are consistent with the observation that cruciate disease tends to occur at a younger age in large-breed dogs.44 The exact cause of cranial cruciate ligament rupture is poorly understood. Factors that have been implicated include abnormal conformation and gait,26 increased tibial plateau angle,223,256,293,326 obesity,209,363 and lack of fitness44; however, none of these has proved causative. For instance, the mean tibial plateau angle was reported to be significantly greater in dogs with cranial cruciate ligament rupture than in dogs with an intact cranial cruciate ligament.256 However, additional investigations failed to substantiate this finding.297,381 A retrospective study revealed that neutering increases the prevalence of cranial cruciate ligament injury.320 It has been shown that the cranial cruciate ligament is rich in mechanoreceptors and proprioceptors,18,388 and that joint loads that cause increased strain in the cranial cruciate ligament result in simultaneous contraction of the caudal thigh muscles and relaxation of the quadriceps muscle group,242,329 which is protective of the cranial cruciate ligament. Thus, it is possible that obesity and/or poor physical condition may mitigate these protective mechanisms, resulting in repetitive strain injury of the cranial cruciate ligament, leading to mechanical failure.300,329 Histologic examination of ruptured cranial cruciate ligaments revealed lack of collagen fiber maintenance363 and loss of fibroblasts from the core region, despite a normal cell number density in the epiligamentous (thin layer of connective tissue covering the ligament) region.152 The decline in fibroblast numbers was further characterized by a decrease in the numbers of typical fusiform and ovoid fibroblasts, an increased number of cells undergoing chondroid metaplasia, and extensive disruption of the ligamentous matrix.152,363 Decreased birefringence and elongation of crimping in the remaining collagen fibrils were identified, which implicates progressive mechanical overload as a cause of failure.152 A proliferative epiligamentous repair response was identified that resulted in eventual covering of the torn ends of the ligament. However, no bridging scar was identified between the ends.152 Factors that may contribute to this degeneration include immune-mediated degeneration,137,261 acquired loss of blood supply in the midportion of the ligament,363 and a smaller intercondylar notch width.6 In simplistic terms, the ligament is too weak to withstand the forces applied to it, or the forces applied are greater than the strength of the ligament. In all likelihood, various interrelated factors contribute to rupture of the cranial cruciate ligament in the dog. The extracellular matrix of ruptured cranial cruciate ligaments has been shown to have an increased matrix turnover, demonstrated by increased collagen and glycosaminoglycan synthesis, compared with intact cranial cruciate ligaments.80 Antibodies to type I and II collagen have been found in the sera and synovial fluid of dogs with spontaneous cranial cruciate ligament rupture, leading to speculation that immunologic reactivity may play a role in cruciate ligament disease.261 However, in a more recent study comparing the prevalence of antibodies to collagen types II and I in the synovial fluid of dogs with cranial cruciate ligament disease, and dogs with osteoarthritis secondary to other pathologic processes, the authors concluded that the increase in synovial fluid antibodies was not specific for the type of joint disorder. In addition, the investigators suggested that it is unlikely that the anticollagen antibodies play an active role in the initiation of weakening of the cranial cruciate ligament.98 Breed variation in the material properties of the cranial cruciate ligament has been reported. One in vitro investigation comparing the cranial cruciate ligament from the Rottweiler with that of the racing Greyhound revealed that Rottweiler ligaments had a significantly greater cross-sectional area at their tibial attachment. Mechanical testing of these ligaments showed that the ultimate failure load, normalized to body mass, was significantly greater in the extended racing Greyhound stifle during loading in cranial tibial subluxation. The conclusion from this work was that the Rottweiler ligament is more vulnerable to damage because it requires half the load per unit body weight to rupture compared with the Greyhound ligament.385 Cranial cruciate ligament disease affects a wide variety of dog breeds; in one study, the highest prevalence was seen in the Rottweiler, Newfoundland, and Staffordshire Terrier, and the lowest prevalence in affected breeds was noted in the Dachshund, Basset Hound, and Old English Sheepdog.377 In another study of dogs that sustained cranial cruciate ligament rupture before 2 years of age, predisposed breeds included the Neapolitan Mastiff, Akita, Saint Bernard, Rottweiler, Mastiff, Newfoundland, Chesapeake Bay Retriever, Labrador Retriever, and American Staffordshire Terrier.112 Female dogs have an increased prevalence of cruciate ligament disease compared with male dogs,377 and in both studies, neutered dogs had a higher prevalence than sexually intact dogs.112,377 However, age at the time of ovariohysterectomy was not associated with the prevalence of cruciate disease.377 Several studies have documented that smaller dogs—those weighing less than 22 kg—tend to be affected later in life than larger dogs.37,44,365,377 This epidemiologic finding is consistent with histologic analyses revealing that the cranial cruciate ligaments of dogs weighing less than 15 kg generally showed less severe degeneration of the ligament than those of larger dogs, and onset of the degenerative process was delayed by several years in the smaller dogs.363 Rupture of the contralateral cranial cruciate ligament occurred in 37% of dogs at a mean of 17 months following initial diagnosis of unilateral cranial cruciate ligament rupture; the remaining 63% had not ruptured their contralateral cranial cruciate ligament at a mean follow-up period of 31 months in one study.106 In another study, 48% of Labrador Retrievers ruptured the contralateral cranial cruciate ligament at a median time of 5.5 months.61 Tibial plateau angle was not found to be a useful predictor of contralateral rupture in dogs with unilateral cranial cruciate ligament disease,68 and in the Labrador Retriever, age, tibial plateau angle, sex, and weight did not appear to be risk factors for contralateral rupture.61 Physical examination reveals stifle pain with flexion and extension, variable crepitus, and possible clicking associated with a meniscal tear (meniscal click). In cases of partial tear of the cranial cruciate ligament, a pain response is commonly elicited when the stifle joint is placed in full extension. In chronic cases, quadriceps muscle atrophy is notable, and periarticular fibrosis is most evident on the medial side of the stifle. This medial periarticular hypertrophy has been referred to as medial buttress formation. Stifle joint effusion is often noted by the absence of clearly defined, sharp borders on the medial and lateral aspects of the patellar ligament. Dogs will often sit with the affected leg projecting out to the side, rather than sitting squarely (fully flexing the stifle joint, with the hock, stifle, and hip in the same plane [such as a sitting Sphinx] and the tuber calcanei in close proximity to the ischiatic tuberosity); this has been termed an abnormal “sit test.”323 Disorders of the hock may also result in an abnormal sit test; thus careful physical examination is warranted to ensure that abnormalities of the hock do not exist or coexist. In cases of partial tear of the cranial cruciate ligament, cranial drawer may be present or absent.311,342 If the craniomedial band is torn and the caudolateral part is intact, cranial drawer is present in flexion only because the intact caudolateral part is taut in extension. If the caudolateral part is torn and the craniomedial band is intact, no cranial drawer is present because the craniomedial band is taut in both flexion and extension. Mild effusion of the stifle joint, pain when the stifle is held in extension, and discomfort noted during the cranial drawer test are consistent with partial cranial cruciate ligament rupture; these findings are useful in confirming that the cause of the lameness is related to the stifle joint, even in the absence of discernible cranial drawer. In cases of stable partial tears of the cranial cruciate ligament, alternative diagnostic methods such as radiography, magnetic resonance imaging (MRI,), and arthroscopy can be used to confirm the presence of stifle pathology and partial tear of the ligament. The tibial compression test is another test of stifle joint stability in the sagittal plane; in this test, the operator creates stifle joint compression that results in a cranial tibial thrust force. The intact cranial cruciate ligament counters cranial tibial thrust; however, if the ligament is ruptured, cranial tibial translation occurs. To perform this test, the examiner is usually located caudal or caudal and lateral to the patient. The patient can be standing or in lateral recumbency with the affected limb uppermost. The index finger of one hand is placed on the tibial tuberosity, and the thumb, palm, and remaining fingers of that hand are used to grasp the femoral condyles and to maintain stifle joint extension. The index finger is used to press caudally on the tibial tuberosity, reducing the tibia into a neutral position if it was cranially subluxated. The other hand is used to grasp the metatarsus, and the tarsocrural joint is alternately flexed and extended, simulating contraction of the gastrocnemius muscle and thus the tibial compression mechanism. The tibial tuberosity is monitored with the index finger for cranial-to-caudal motion in the sagittal plane.157 The presence of motion suggests compromise of the cranial cruciate ligament.
Stifle Joint
Anatomy, Structure, and Function118
Bones of the Stifle Joint118
Ligaments of the Stifle Joint
Meniscus
Kinematics of the Normal Stifle Joint
Meniscal Function
Effect of Meniscal Release on Meniscal Function
Kinetics and Kinematics of the Cranial Cruciate Ligament–Deficient Stifle Joint
Theoretical Models of Stifle Joint Stability and Instability
Cranial Cruciate Ligament Disease
Avulsion of the Cranial Cruciate Ligament
Treatment Considerations
Acute Traumatic Rupture of the Cranial Cruciate Ligament
Progressive Degeneration of the Cranial Cruciate Ligament
Physical Examination
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stifle Joint
Only gold members can continue reading. Log In or Register to continue