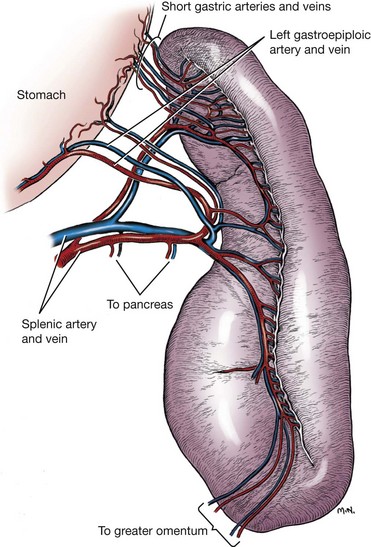
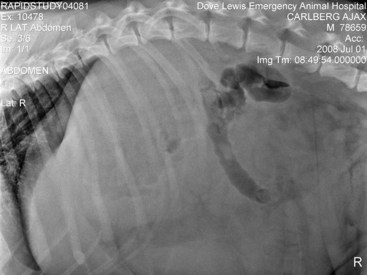
Chapter 83 The spleen is a dynamic falciform organ usually residing in the left cranial quadrant of the abdomen. It has been described as red, reddish-brown, purple, mahogany, and gray-brown with a purple cast.21,22,29 A normal spleen is soft and yielding in consistency but not as friable as the liver and is approximately 0.2% of the body weight in dogs and cats.29 The dorsal extremity (head), tethered to the greater curvature of the stomach by the wide gastrosplenic ligament, is generally narrower than the ventral end and fits loosely into the space defined by the left kidney, left crus of the diaphragm, and gastric fundus. The larger ventral extremity (tail) is quite mobile but generally lies across the ventral midline just caudal to the ribs. The parietal surface of the spleen is convex, and the visceral surface is concave, with a longitudinal ridge marking the attachment of nerves, vessels, and omentum. In cross-section, the spleen appears triangular. The splenic vascular supply arises from the celiac artery, which is the first unpaired ventral branch of the abdominal aorta. The celiac rapidly divides into three branches: the hepatic, left gastric, and splenic arteries.3 Rarely, the left gastric and splenic arteries arise from a short common trunk. The splenic artery runs the length of the left limb of the pancreas, giving rise to the pancreatic artery before angling to the hilus of the spleen (Figure 83-1). The pancreatic artery may be supplemented by up to three additional branches from the splenic artery. Off of the cranial surface of the splenic artery arise one or two long vessels that angle toward the cranial extremity and ultimately course through the gastrosplenic ligament to supply the short gastric arteries. The cranial half of the spleen is supplied by several branches off these long vessels. The short gastric arteries ultimately anastomose with the branches of the left gastric artery. After it passes the middle of the spleen, the continuation of the splenic artery is called the left gastroepiploic artery. It gives off several splenic branches to supply the caudal splenic extremity and angles back to the greater curvature of the stomach through the gastrosplenic ligament. The splenic vein collects blood from the many hilar veins and drains into the gastrosplenic vein before entering the portal vein. Splenic lymph nodes can be found near the splenic vein and artery a few centimeters distant from the hilus.21 The parenchyma of the normal spleen consists of red and white pulp surrounded by a capsule and traversed by a complex network of trabeculae.22 Whereas red pulp stores erythrocytes and traps antigens and is the site of fetal erythropoiesis, white pulp is the site of immune response.82 Splenic capsule is made of elastic and smooth muscle; the trabeculae are formed from a complicated network of fibromuscular fibers. White pulp consists of diffuse and nodular lymphoid tissue. Nodules have a similar microscopic structure to lymph nodes, with a primary follicle at the center. Diffuse lymphoid tissue adjacent to splenic arteries, known as periarteriolar lymphatic sheaths, contain primary follicles of B-cells surrounded by a mantle of T-cells that serve to process antigens filtered from the blood. Red pulp contains the venous sinuses and cellular tissue between sinuses. Lymphocytes, macrophages, and all circulating blood cells are found in this loose meshwork of endothelial cells. Activated B-cells are returned to the circulation via the venous sinus system. Asplenia, the failure of the spleen to develop in utero, is an occasional occurrence in dogs and cats.26 More common is the presence of small fragments with a normal splenic architecture on histopathologic examination. The term accessory spleen has been applied to congenital and acquired fragments, although fragmentation and subsequent survival of splenic tissue after trauma is more appropriately termed splenosis. Congenital accessory spleens are seen in all species and can be found throughout the body, including the thoracic cavity, inguinal canal, and scrotum. The most common site in dogs is within the gastrosplenic ligament. These are generally benign findings unless acting as space-occupying lesions. Splenic fissures are a common finding in dogs. These developmental defects can be differentiated from healed lacerations by the normal appearance of the capsule and smooth surface within the fissure. During fetal development, the splenic red pulp is a major site of extramedullary hematopoiesis. In the early neonatal period, RBC production shifts primarily to bone marrow. Adult animals can reinstitute splenic hematopoiesis when significant physiologic demand results in hypoxia. The spleen is responsible for the final maturation of RBCs before release into circulation. After production in the bone marrow, RBCs spend several days in the spleen, where intracellular material is removed, the cell membrane is shaped into a disc, and cell size reduced. In a similar fashion, damaged or senile RBCs are culled from circulation for a variety of reasons. Inelastic cells such as spherocytes or acanthocytes are unable to deform adequately to pass through either the sinusoidal or nonsinusoidal spleen and are consequently phagocytosed by reticuloendothelial cells (fixed macrophages). RBCs covered by immunoglobulin or with intracellular bacteria are also triggers for filtration by macrophages. Iron from culled RBCs is stored in the spleen as ferritin and hemosiderin until transported to the bone marrow for hematopoiesis. Splenic extramedullary hematopoiesis occurs during fetal growth and the neonatal period until bone marrow takes over the responsibility. In adult animals, the spleen can resume some RBC production in response to infiltrative diseases of the bone marrow or with increased demand secondary to peripheral RBC destruction. Splenic extramedullary hematopoiesis can manifest as generalized splenomegaly or as focal nodules and is a rare finding in cats.48 Single or multiple splenic nodules with active hematopoiesis on histologic examination are common in older dogs. The spleen can store 10% to 20% of a dog’s RBC mass and 30% of the platelet mass.48 Blood drawn from the splenic sinus system has a very high hematocrit (80% to 90%) to accommodate this storage. RBCs passing through the spleen during normal circulation are divided into three “pools,” depending on the circulatory pattern. The rapid pool accounts for approximately 90% of blood entering the spleen and takes less than 30 seconds to rejoin the systemic circulation. The intermediate pool (9% of circulating blood) takes 8 minutes to traverse the spleen, and the slow pool (1% of circulating blood) takes 1 hour. Physiologic demand mediates splenic contraction via circulating pressors and direct nerve action on splenic smooth muscle, resulting in up to 98% of stored erythrocytes moving into the rapid pool and reducing splenic size to 25% to 50% of normal. Splenitis refers to an inflammatory infiltrate that can be further classified by the predominant cell type. It has been associated with bacterial, viral, fungal, and protozoal exposure.69 The most common causes of uniform splenomegaly in the dog include bacteremia, low-grade septicemia, and chronic infectious disease in which pathogens and necrotic debris are filtered in the spleen.69 Infectious splenitis is dominated by a neutrophilia, which can progress to a localized abscess in some cases. Cats with hypereosinophilic syndrome and dogs with eosinophilic gastroenteritis may present with an eosinophilic splenitis in addition to other clinical signs. Pyogranulomatous splenitis has been described in cats with feline infectious peritonitis.33 Lymphoplasmacytic infiltrative splenomegaly is seen with chronic or subacute infection and mycotic or mycobacterial infection. Necrotizing splenitis develops with gas-forming bacteria. Hypercoagulative states resulting in thrombosis of splenic vasculature and subsequent seeding of the hypoxic regions with Clostridia spp. have been described. Coagulative necrotic infectious agents are rare in dogs and cats, most likely because of substantial blood flow and oxygen supply in both the red and white pulp regions. In many cases, generalized splenomegaly results from proliferation of normal cellular components as the spleen performs its regular functions. In animals affected by chronic disease, the spleen may have a markedly enlarged marginal zone of white pulp; however, cellular hyperplasia may occur in the red pulp, white pulp, or both. Subacute and chronic diseases such as immune-mediated hemolytic anemia and immune-mediated thrombocytopenia generally result in cellular hyperplasia of white and red pulp. Splenomegaly is common in dogs with immune-mediated hemolytic anemia and results from extramedullary hematopoiesis and reticuloendothelial hyperplasia associated with destruction of IgG-covered erythrocytes.69 Macrophage hyperplasia, which occurs with increased phagocytic activity, primarily affects red pulp and is commonly seen in histoplasmosis and leishmaniasis. Cell-mediated and humoral immunity is activated by chronic antigenic stimulation of T- and B-lymphocytes of white pulp, as seen with osteomyelitis or bacterial endocarditis. Lymphoid hyperplasia of the spleen can also present as nodular rather than generalized in response to chronic stimulation. Splenic myeloid metaplasia with histiocytosis has been described in 65 dogs with generalized splenomegaly.78 The prognosis with this diagnosis was poor, with 70% of dogs deceased 1 year after splenectomy. Congestive enlargement affects the entire spleen and is usually the result of one of four mechanisms: congestive heart failure, portal hypertension, vascular outflow obstruction, or relaxation of the splenic capsule in response to chemical stimuli. Barbiturates, such as thiopental and phenobarbital, and phenothiazine tranquilizers relax splenic capsular smooth muscle, allowing blood to pool within sinusoids. Other commonly used premedication, induction, and anesthetic maintenance drugs may directly affect circulating RBC volume and thus splenic size; however, interaction and synergy of perioperative medications makes intraoperative interpretation of spleen size challenging.89 Because the feline spleen has a nonsinusal design, severe splenomegaly is less likely to be physiologic in origin in that species.33 Vascular outflow obstruction in the form of acute splenic torsion or portal vein or caudal vena cava obstruction will result in passive congestion of the spleen and generalized splenomegaly.48 Passive congestion may also occur with intraabdominal masses, such as abscess, neoplasms, and adhesions, that alter venous drainage. Gastric dilatation and volvulus (GDV), without additional torsion of the splenic pedicle, frequently results in splenic congestion from venous compression and stretching. Splenic infiltration by abnormal cells or substances is seen in neoplastic processes (primary and metastatic) and, rarely, in splenic amyloidosis. Generalized splenomegaly of neoplastic origin can occur with primary neoplasia arising from cell populations that normally exist in the spleen, including lymphocytes, macrophages, fibroblasts, smooth muscle, or endothelium. The most common neoplastic cause of generalized splenomegaly is myeloproliferative neoplasia, such as lymphosarcoma and mastocytosis.26 Splenic histiocytic sarcoma, which results in generalized and focal splenic enlargement, has been described in several dogs. Flat-coated retrievers, Rottweilers, Bernese mountain dogs, and golden retrievers are considered to have an increased risk for this condition.20 Metastatic disease is uncommon in the spleen and is usually focal or multifocal rather than generalized. Lymphoma is the most common metastatic lesion of the spleen.39 Rarely, lysosomal storage diseases and splenic amyloidosis may cause generalized splenomegaly. Lysosomal storage diseases are inherited metabolic defects that result in accumulation of lipids, carbohydrates, or both. Macrophages in the spleen are unable to process the substrates and consequently store them in an unprocessed form. These rare diseases are usually diagnosed in animals younger than 1 year of age and result in a uniformly enlarged, firm spleen that may be pale because of stored lipids.26 In dogs with splenic amyloidosis, the spleen appears pale beige, firm, and waxy. Nodular hyperplasia, historically called splenoma, is most common in older dogs and is often an incidental finding. Nodules are composed of lymphoid, erythroid, myeloid, and megakaryocyte cells. They can occur regionally or focally in the parenchyma as discrete or coalescing, firm, subcapsular nodules protruding from the splenic surface. Hyperplastic nodules are usually benign. Some authors suggest, however, that they may progress to hematomas because of failure of marginal zone circulation, which could lead to accumulation of blood within the nodule. Histologically, splenic hematomas and hyperplastic nodules are distinct but may represent a continuum in dogs.48,75 Nodular hyperplasia is less common in cats, perhaps because the nonsinusoidal nature of the spleen in cats creates less opportunity for venous pooling. In dogs, a fibrohistiocytic nodule is a histologically distinct type of nodular hyperplasia.26,76 This condition is considered an intermediate phase between nodular hyperplasia and malignant fibrous histiocytoma. Cocker spaniels are reported to have an increased incidence of fibrohistiocytic nodules. Other reported breeds include German shepherds, Labrador and golden retrievers, and standard poodles. Fibrohistiocytic nodules, nodular hyperplasia, and neoplastic nodules cannot be differentiated grossly at surgery. Splenic inflammatory pseudotumor is a rare condition described in dogs and humans.28 This benign lesion is composed predominantly of plasma cells, lymphocytes, and histiocytes in a fibroproliferative background and thus must be differentiated from nodular hyperplasia and malignant tumors such as lymphoma and sarcoma. In humans, the prognosis is good after splenectomy. The prognosis is unknown in dogs because of the rarity of this lesion; the single documented canine case was doing well months after surgery. Benign canine hemangioma has been documented in several patients with localized splenomegaly. These are usually solitary masses composed of well-differentiated endothelial cells that connect to well-formed vascular spaces.18,26 This tissue structure separates hemangioma from hemangiosarcoma, in which endothelial cells and vascular spaces are haphazardly arranged. However, hemangioma, hematoma, and hemangiosarcoma all have a similar, if not identical, gross appearance in dogs. Canine splenic hamartomas represent a rare benign proliferation of mature cells and tissues that are normally present in the spleen, similar to nodular hyperplasia.53 However, hamartomas differ in that they do not reproduce the normal architecture of the surrounding tissue. They are impossible to differentiate from other forms of localized splenomegaly on routine ultrasonography, although there are no reports regarding the use of contrast-enhanced ultrasonography with this specific lesion. Both computed tomography (CT) and magnetic resonance imaging (MRI) have been used in human medicine to better differentiate hamartomas from other benign masses. Splenic abscesses are uncommon in dogs and rare in cats. They are generally associated with other conditions that compromise vascular supply or drainage of the spleen. Torsion of the vascular pedicle resulting in congestion, hypoxia, and necrosis of the splenic parenchyma may lead to abscess formation. Uncommonly, splenic foreign body has been described as the cause of discrete abscess.16 Some microorganisms that typically result in generalized splenomegaly can also cause focal lesions (chronic suppurative splenitis). In septic animals with bacteremia, bacterial agents filtered by the spleen that are not killed may be capable of replication within the parenchyma. Segmental splenic infarction is uncommon in dogs, representing 1% to 2% of lesions found histopathologically.75 Infarction occurs primarily in subcapsular areas, possibly as a result of poor perfusion and reduced venous return (Figure 83-2). Infarcts also form in areas of acute vascular occlusion secondary to infectious agents. For example, with hemobartonellosis (Mycoplasma haemocanis infection), abnormal cells extracted from blood overwhelm splenic circulation, resulting in congestion and ultimate blockage of small vessels. Segmental infarcts are generally wedge shaped, with the base at the periphery, but can also be nodular or can involve an entire extremity.26 On cut surface, whereas acutely infarcted areas are red-dark purple, chronic lesions may be gray-white to tan. Global and segmental splenic infarctions secondary to pancreatitis have been described only in the human literature.59 Figure 83-2 Segmental splenic infarcts in an 11-year-old Swiss mountain dog with intestinal lymphosarcoma. CT scan with intravenous non-ionic contrast and MRI with intravenous gadolinium contrast are sensitive techniques for the diagnosis of splenic infarction. Ultrasonographic examination may reveal focal, hypoechoic, or isoechoic well-marginated lesions that deform splenic contour or a diffuse hypoechoic or heterogenic parenchymal pattern that does not affect the splenic contour.34 A retrospective study of 16 dogs with splenic infarction identified several predisposing causes, including hypercoagulable states, splenomegaly, cardiac disease, neoplasia, liver or renal disease, excessive corticosteroids (endogenous or exogenous), sepsis, splenic hematoma, and vasculitis.34 Dogs in which a coagulation profile was performed had evidence of a coagulopathy, such as increased prothrombin time, partial thromboplastin time, or fibrin degradation products or thrombocytopenia. Because of concurrent hypercoagulability, splenectomy in affected dogs is associated with increased postoperative morbidity and mortality. Therefore, segmental splenic infarction alone is not an indication for surgery. Siderotic plaques are benign golden brown or black patches that are frequently seen on the surface of the spleen. They result from focal accumulations of stored iron (hemosiderosis) derived from erythrophagocytosis and subsequent hemoglobin breakdown.26,69 Whereas capsular hemosiderosis occurs as a result of hemorrhage, parenchyma iron accumulation may occur secondary to hemorrhage, hematoma, or infarcts. Siderocalcific plaques (or Gamma-Gandy bodies) are whitish to yellowish, dry encrustations on the margins of the spleen; occasionally, they are also found in parenchyma. Histologically on hematoxylin and eosin stain, they contain golden brown hemosiderin (similar to siderotic plaques) but with the addition of yellow and blue areas from presence of bilirubin and calcium, respectively. Siderocalcific plaques are considered a senile change but may be associated with previous splenic hemorrhage.26,69 Neoplastic causes of localized splenomegaly can be divided into hemic and nonhemic sources. Hemic neoplasms include lymphoid, mast cell, histiocytic, plasma cell, and myeloproliferative disease. Concurrent evaluation of peripheral blood, bone marrow, and other hemic organs is critical for accurate diagnosis and staging of affected patients. Nonhemic neoplasms include hemangiosarcoma, other sarcomas, and benign tumors of connective tissue origin. Because of the cavitary nature of hemangiosarcoma, neoplastic endothelial cell proliferation can be difficult to identify. This makes differentiation of hemangiosarcoma, hemangioma, and hematoma challenging. In general, splenic neoplasia is more commonly localized in dogs and generalized in cats. Splenic neoplasia is discussed in further detail below (see Surgical Conditions of the Spleen). The spleen in dogs and cats is easily visualized radiographically; however, its location is variable because of mobility of its caudal aspect. A standard ventrodorsal projection typically shows the cranial extremity of the spleen as a triangular cross-section in the left cranial quadrant caudolateral to the gastric fundus and craniolateral to the left kidney.46 The caudal extremity often extends along the left body wall; however, it may be obscured because of summation with other viscera if it is more axially located. The spleen in dogs is seen more consistently on the right lateral view than the left lateral. In a lateral projection, the silhouette of the splenic caudal extremity appears as a triangular, oval, or rounded structure slightly caudal and ventral to the pylorus or liver. Thinner and smaller, the feline spleen is seen in ventrodorsal and lateral projections in the same orientation as the dog spleen, although the caudal extremity may not be visible in the lateral view.46 Large splenic masses often appear in mid-abdomen on ventrodorsal and lateral views (Figure 83-3). Masses of the caudal extremity or body of the spleen tend to displace small intestines dorsally and caudally. Cranial displacement of the stomach and caudal, medial, and ventral displacement of the intestines are suggestive of a cranial splenic mass. Generalized splenomegaly results in rounded or blunted splenic margins. Hemoabdomen from splenic hemorrhage or rupture may reduce radiographic visceral detail, making it difficult to identify the splenic and hepatic silhouettes. Ultrasonography is the diagnostic modality most frequently used to identify abnormal splenic architecture and screen the abdomen for metastatic disease.24 During ultrasonography, the splenic contour should be examined for irregularities such as focal enlargement or disruption, as is seen with trauma, hematoma, and some neoplastic lesions. The normal splenic capsule appears as a smooth, regular, strongly echogenic line that curves gently down the length of the splenic body. The parenchyma is inspected for homogeneity and the vasculature for evidence of flow. Splenic size is evaluated; however, there are no minimum or maximum normal measurements for judging splenic size in cats and dogs; consequently, experience is critical in interpreting findings. Because of its nonsinusal architecture, the spleen in cats is unlikely to be enlarged for physiologic reasons, and it is rare to see a cat’s spleen folded ultrasonographically unless it is enlarged.33,46 Compared with the liver, the spleens in cats and dogs exhibit finer echotexture and are more echogenic. Whereas hypoechoic nodules within the spleen are associated with lymphoid infiltration and with infarction and necrosis, diffusely hypoechoic parenchyma is often seen with passive splenic congestion and splenic torsion. Hyperechoic changes are consistent with nodular hyperplasia, primary or metastatic focal neoplasms, and fibrosis from healed infarction or hematoma. In one study, detection of target lesions—nodules with hypoechoic rim and hyperechoic or isoechoic centers—had a positive predictive value for malignancy.15 Whereas spleens with multiple discrete lesions of similar ultrasonographic appearance are significantly associated with malignancy, single lesions are frequently benign.4 Hematomas, neoplasms, abscesses, and cysts may all appear as focal cavitary lesions. Contrast-enhanced ultrasonography improves characterization of focal and multifocal lesions of feline and canine spleens.70 Peripheral injection of a small quantity of microbubble contrast medium allows comparison of perfusion of focal lesions with surrounding parenchyma. This technology is used routinely in Europe for characterization of liver lesions and compares favorably with the accuracy of contrast-enhanced MRI and CT.54 In a recent study in dogs, most benign splenic lesions had a perfusion pattern similar to normal parenchyma, but malignant lesions had a different pattern than adjacent parenchyma. Although contrast-enhanced ultrasonography is portable, rapidly performed, and less expensive than CT or MRI, there is not yet enough published experience in veterinary patients to fully institute its use. When using diagnostic ultrasonography in patients with splenic disease, it is important to evaluate the remainder of the abdomen for effusion, lymphadenopathy, hepatic abnormalities (hepatomegaly, diffuse change in echogenicity, nodules), and intestinal thickening or masses. Up to 25% of dogs with splenic hemangiosarcoma may also have right atrial hemangiosarcoma, making cardiac ultrasonography before surgery a worthy investment.85 CT and MRI are gaining popularity in veterinary medicine with respect to splenic disease. Both modalities are used routinely in human medicine for solid organ evaluation and are particularly useful in identification of concurrent underlying disease.66,67 Although the use of advanced imaging is limited by cost and availability, some recent studies suggest that CT and MRI may provide useful information in stable patients, including evaluation of splenic masses and identification of abdominal or thoracic metastases. A recent prospective study evaluating contrast-enhanced CT of splenic lesions reported that splenic hemangiosarcoma had significantly lower density (lower Hounsfield units) than nodular hyperplasia or hematomas on pre- and postcontrast images.23 In another prospective study, benign and malignant lesions were accurately differentiated in eight of eight dogs using MRI.13 In that study, malignant disease was hyperintense in T2 and postgadolinium phases of the exam, and benign lesions were hypointense.13
Spleen
Anatomy
Physiology
Hematopoiesis
Reservoir Function
Pathology
Splenitis or Inflammation
Immune Reaction or Cellular Hyperplasia
Congestion
Infiltration
Localized Splenomegaly
Nodular Hyperplasia
Pseudotumor
Hemangioma
Hamartoma
Abscess
Segmental Infarction
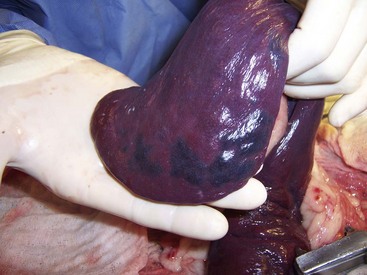
Plaques
Neoplasia
Diagnostic Imaging Techniques
Ultrasonography
Computed Tomography and Magnetic Resonance Imaging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree