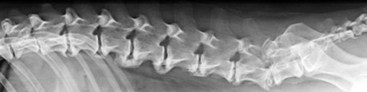
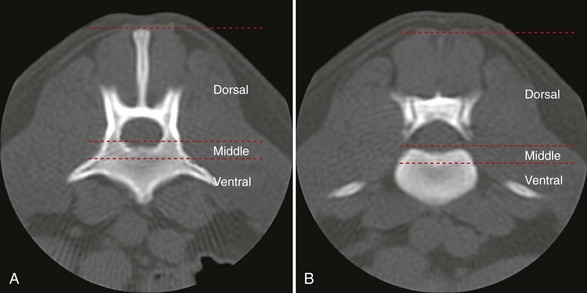
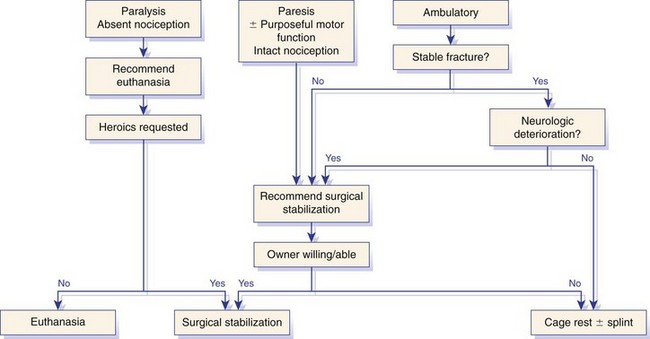
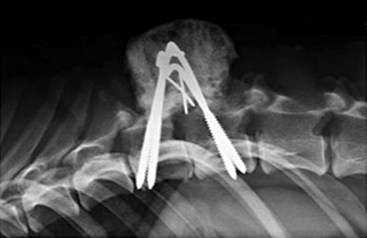
Chapter 34 Fractures and luxations of the spinal column are causes of spinal cord injury in dogs and cats. Depending on the location and severity of injury, subsequent neurologic dysfunction ranges from pain to tetraplegia. Spinal fractures and luxations are typically the result of major physical trauma, most commonly vehicular injury and falls from heights. Injuries due to animal attacks, gunshot wounds, and other causes are also reported.* The incidence of spinal fracture among dogs treated for severe blunt trauma is reported to be 10%.92 Motor vehicle accident is the cause of injury in 41% to 63% of these cases.6,23,46 Less frequent, nontraumatic causes of spinal fractures and luxations include neoplasia, infection, and metabolic disease.27,79,86 Every region of the spine is susceptible to injury, and the most commonly affected site varies between studies.67,69,84,94,103 The two most recent retrospective studies found the thoracolumbar spine (T3-L3) to be most often affected, followed by the lumbar spine (L4-L7).6,23 Some authors28,83 have argued that the thoracolumbar and lumbosacral junction is predisposed to injury because of stress concentration at the junction of relatively mobile and immobile spinal segments. However, this has not been supported by other studies.6,23,103 The pathophysiology of acute spinal cord injury has been reviewed elsewhere,60,74,78 and the reader is directed to Chapter 29 for a detailed discussion of relevant mechanical, vascular, and molecular factors contributing to spinal cord injury and dysfunction. Broadly speaking, spinal cord injury can be defined as primary or secondary. Primary injury is a mechanical insult that usually can be classified as concussion, compression, shearing, laceration, or elongation. The spinal cord may be subject to multiple primary injuries subsequent to the initial hit, because of ongoing instability or persistent compression. The mechanical insult directly damages nervous tissue and induces a cascade of vascular and molecular events, leading to secondary changes such as hemorrhage, ischemia, and edema. A cycle of secondary neuronal injury is induced and mediated via factors such as free radicals, excitatory neurotransmitters, cytokines, inflammatory mediators, ionic dysregulation, and catecholamines. This secondary injury may be as damaging as the primary injury itself,53,74,78 and the severity of overall spinal cord damage is a reflection of both primary and secondary injuries. Trauma Assessment and Stabilization Most spinal fracture patients are victims of trauma, and 45% to 83% of them have concurrent injuries.6,23 The initial goal is to stabilize these patients by treating shock and life-threatening injuries. Providing hemodynamic resuscitation to the patient in shock not only supports the cardiovascular system, but also ensures adequate oxygenation and perfusion to the spinal cord, helping to minimize further neuronal damage and deterioration. Depending on the patient’s condition, spinal injuries may not become apparent until after initial assessment and stabilization. Obtunded animals provide a challenge for performing an accurate neurologic examination. Likewise, focusing only on an obvious spinal injury may result in other serious problems being overlooked. An efficient and thorough history and physical examination should be performed according to standard emergency protocol, and a standard minimum database of tests should be obtained. As with all trauma cases, some injuries may not be apparent on initial examination, and owners should be advised of this. If spinal cord injury is suspected, the animal should be immobilized on a board during initial assessment and stabilization. Spinal cord injuries are dynamic, and neurologic status may worsen if immobilization is delayed.46,69 For suspected thoracolumbar injury, the patient can be secured in lateral recumbency to a rigid surface by straps or tape across the pelvis and scapula. When cervical injury is suspected, the head should be immobilized as well. Pharmacologic sedation and pain relief can aid in immobilization but should be administered only after the initial trauma and neurologic assessment has been completed, to avoid interference with patient assessment. Thoracic trauma is common and is reported in 15% to 35% of cases.23,103 Injuries include pulmonary contusions, rib fractures, and pneumothorax. Pulmonary contusions can worsen in the 24 to 48 hours after trauma, and may be a significant concern for anesthesia. In addition to thoracic auscultation, thoracic radiographs are indicated to rule out these injuries. Electrocardiography should also be performed to rule out cardiac arrhythmias that may require treatment. Abdominal trauma is reported in 6% to 15% of spinal fracture cases23,103 and may result in hemoperitoneum, uroperitoneum, or gastrointestinal or hepatobiliary injury. Minimum laboratory database usage and focused ultrasound examination in the emergency setting can help to rule out these injuries at an early stage.18 Concurrent fractures are common, occurring in 14% to 48% of cases.23,103 Pelvic fractures or fractures of the appendicular skeleton can be confounding factors when assessing neurologic status in spinal fracture patients. In these cases, it is very important to determine whether clinical dysfunction is due to neurologic or musculoskeletal causes, especially when predicting prognosis for neurologic recovery. Multiple spinal fractures or luxations have been reported in 15% to 20% of cases, and may not occur at adjacent vertebrae or even within the same neuroanatomic region. Dogs weighing less than 15 kg may be more predisposed to multiple spinal fractures than larger dogs.6 Survey spinal films of the entire spine are recommended to rule out injury at a distant site (Figure 34-1). A complete neurologic examination is critical for localizing spinal cord lesion(s), detecting concurrent neurologic disease, and prognosticating outcome. It is important to perform a full examination and avoid focusing solely on the region of known injury. Details of the comprehensive neurologic examination are discussed in Chapter 26. Patients with spinal fractures exhibit a range of neurologic deficits similar to those seen in any patient with acute spinal cord injury: proprioceptive deficits, voluntary motor deficits, alterations in spinal reflexes, and sensory deficits. Evaluation of voluntary motor function or ambulation may be hampered if the patient is already immobilized on a board. Speaking to or touching the animal may elicit voluntary limb or tail movement. In addition, owner history may confirm movement or ambulation after the traumatic event. It is important to remember that in the case of multiple spinal injuries, the signs of one lesion may mask those of a second lesion. For example, a lesion in the cervical intumescence (C6-T2) may cause lower motor neuron signs to the forelimbs and upper motor neuron signs to the hindlimbs. The upper motor neuron signs caused by a thoracolumbar (T3-L3) lesion could be indistinguishable. Conversely, a lumbosacral (L4-L7) lesion would be expected to cause lower motor neuron signs to the hindlimbs, making it difficult to detect a more cranial thoracolumbar (T3-L3) lesion. The most important prognostic factor for recovery following spinal cord injury is the presence of nociception, that is, the ability to sense a noxious stimulus. The absence of nociception in the limbs caudal to the spinal cord lesion indicates a poor prognosis for return of function to the affected limbs. Relatively few studies have directly examined the prognosis for recovery in animals with traumatic spinal cord injury and lack of nociception. Because data supporting successful treatment of these patients are lacking, most of these cases are euthanized on presentation. Those for whom treatment is initiated are often euthanized because of lack of improvement or complications secondary to paraplegia.6,23,41 One study reported that 2 of 17 (12%) dogs with traumatic thoracolumbar spinal cord injury and lack of nociception at presentation eventually regained the ability to ambulate. However, these patients did not regain normal spinal cord function.74 In contrast, dogs with intact nociception can achieve good outcomes in upward of 80% to 90% of cases.14,69,85 It should be noted that lack of nociception conveys a much worse prognosis for dogs with spinal fracture or luxation than for dogs with intervertebral disc disease. Dogs with intervertebral disc disease who lack nociception at presentation have a chance of a functional recovery of between 60% and 70% in most studies.12,74,82 The prognosis for functional recovery in patients with fracture or luxation is likely at best 12% and may be significantly worse. Some authors have proposed that duration of absent nociception greater than 48 hours worsens the prognosis for functional recovery in dogs with spinal fractures or luxations.5 The paucity of information in the literature regarding these dogs makes this assertion impossible to prove, and the prognosis for dogs with spinal fractures or luxations that lack nociception must be considered poor, regardless of the duration of their condition. The importance of nociception as a prognostic factor dictates its careful assessment in spinal fracture patients. Evaluation of nociception should be done with the animal in as calm and relaxed a state as possible. Ideally, the patient should not be under the influence of narcotic pain medication, as this alters the perception of painful stimuli. Detecting nociception is a rapid test and should not significantly delay the administration of pain relief to a patient in discomfort. It is important to recall that conscious recognition of a painful stimulus is a brain-mediated response rather than a reflex. A simple withdrawal reflex does not indicate intact nociception (see Chapter 26). Signs of conscious recognition of a painful stimulus in these patients may be as obvious as vocalization, turning the head toward the stimulated limb, or attempting to bite; they may be as subtle as an increase in heart rate or respiratory rate, or pupillary dilation. Radiography has limited sensitivity and negative predictive value for detecting osseous lesions in canine spinal trauma. Values of 72% and 48%, respectively, have been reported in a recent study where computed tomography (CT) was used as the gold standard; the actual sensitivity may be lower than 72%.58 Radiography appears to be particularly poor at detecting fractures in the middle and dorsal vertebral compartments, and thus may overestimate the stability of some fractures (see the section on biomechanics). In addition, it is not sensitive for detecting the presence of fracture fragments within the vertebral canal, or spinal cord compression. In the emergency setting, radiography can be used as a screening tool. However, results should be interpreted in light of the test’s relatively poor sensitivity. If a fracture or luxation is detected in the region of the neuroanatomic diagnosis, and neurologic deficits are minor, additional imaging may not be necessary, as these dogs have a very good prognosis with stabilization.86 If a fracture or luxation is detected in a patient with moderate to severe neurologic deficits, additional imaging is strongly suggested to evaluate spinal cord parenchyma, spinal cord compression, and vertebral integrity. Degree of dislocation or axis deviation of the spinal column on radiographs has been negatively associated with outcome in at least one study of dogs.6 However, it is important to recognize that radiographic images provide a static view of a dynamic condition and represent the minimum displacement for any given injury. Lack of displacement at the time of radiography does not eliminate the possibility that severe displacement occurred at the time of injury. Although most authors agree that 100% displacement of the spinal canal on radiographs warrants a poor prognosis for recovery, in all cases the neurologic examination remains the most important prognostic factor. The combination of 100% displacement and absence of nociception warrants a grave prognosis for recovery. Myelography has the advantage over plain radiographs that it can illustrate spinal cord swelling or spinal cord compression due to disc material, hemorrhage, or bone fragments. The diagnosis of a compressive lesion may influence surgical planning to include a decompressive procedure. In addition, dural tears or spinal cord transection may be visible on a myelogram. It is unclear what effect diagnosis of a dural tear has on prognosis, as many of these dogs return to ambulation.47,118 The physical manipulation and additional anesthetic time needed for myelography may cause deterioration of neurologic status and may potentially worsen outcome. The risks must be weighed against the possible benefits. Spinal cord swelling may attenuate the subarachnoid contrast medium over several spinal cord segments. In these situations, myelography may not accurately specify an area of compression or define fracture fragments invading the vertebral canal. For patients with significant neurologic deficits or in which spinal cord compression is suspected, noninvasive imaging techniques such as CT or magnetic resonance imaging (MRI) are preferred over myelography. CT is the imaging modality of choice for diagnosing osseous lesions in spinal cord injury. It has been demonstrated to be significantly superior to radiography for overall diagnosis of spinal fractures and luxations in dogs. Not only does the cross-sectional technique provide more detailed information, but the ability to manipulate the images post facto makes CT more forgiving of patient malpositioning than radiography. This is particularly important in unstable injuries, where manipulation for positioning may cause further trauma. CT is more accurate than radiography in diagnosing multiple sites of injury, and it is particularly valuable in delineating the extent of damage at a given site of injury.58 Although soft tissue structural integrity may not be completely assessed, CT is helpful in evaluating the dorsal, middle, and ventral compartments according to the three-compartment model of spinal stability.32,88 This model allows the most accurate determination of fracture stability and can be used to guide treatment recommendations (see the section on biomechanics). Fracture fragments within the vertebral canal are more readily visualized on CT. These fragments may require removal to prevent risk of further spinal cord or nerve root injury. CT can identify mineralized disc, hemorrhage, and canal narrowing, all of which may contribute to spinal cord compression and may necessitate surgical decompression. The sensitivity of CT for diagnosing compressive lesions can be improved by using subarachnoid contrast material (CT myelography).33 CT can be used, both preoperatively and intraoperatively, to plan and guide implant placement.112 It can also be used with a good degree of accuracy to postoperatively evaluate implant placement with respect to the vertebral canal.48 The major limitations of noncontrast CT are the result of its relatively poor soft tissue resolution. This limits the ability to assess spinal cord parenchyma and may cause underestimation of spinal cord compression. Because of the superior soft tissue resolution of MRI, CT and MRI may best be used in a complementary fashion to evaluate the osseous and soft tissue components of the spinal column and spinal cord.4,62 MRI is the only widely available noninvasive modality that adequately images the spinal cord parenchyma. It is arguably the best method for noninvasive assessment of spinal cord compression. In experimental models, MRI can reliably detect spinal cord edema, hemorrhage, cystic cavitation, and lesion length.62 In addition, it can provide useful information regarding the paraspinal soft tissues, intervertebral discs, and ligamentous supportive structures—all of which contribute to spinal column stability. In human beings and in animals, MRI can be used to diagnose traumatic nerve root avulsions and pseudomeningoceles, as well as sequelae of spinal cord injury such as myelomalacia, syrinx formation, cord tethering, and arteriovenous fistulas.4 Significant research is focused on correlating changes in MRI signal intensity and lesion morphometry with clinical neurologic status and clinical outcome. In human beings with acute spinal cord injury, parenchymal hemorrhage, spinal cord transection, and increased lesion length are all associated with less favorable neurologic outcomes. Lack of MRI signal abnormality within the spinal cord is associated with superior functional recovery. As much promise as MRI may offer for predicting clinical outcomes, when early MRI findings are compared with neurologic examination findings in people, the neurologic examination findings are the single best predictor of outcome.62 In clinical canine intervertebral disc disease, the presence and length of intramedullary T2-weighted signal hyperintensity have been associated with more severe presurgical neurologic grade and worse functional outcome.64,55 These studies have not been performed in dogs with spinal fractures and luxations, but similar associations may exist. Despite the superiority of MRI for spinal cord imaging and advances in MRI bone imaging, CT remains the gold standard for imaging osseous spinal cord lesions. The most sensitive method for evaluating overall vertebral column injury is a combination of MRI and CT.4,62 MRI units still are not widely available for veterinary use, and costs generally are significantly greater than for radiography or CT. MRI scan times are longer than for these other modalities, requiring added anesthesia time for patients. In many cases, patients may have to be moved from their initial immobilization to a position more amenable to the MRI gantry and coil, adding risk in cases of unstable injury. Caution should be used when imaging patients with penetrating spinal cord injury and retained metallic fragments. Although most ammunition is nonferrous, composition is impossible to predict with certainty in clinical cases. Theoretically, a ferrous fragment may become mobile when subjected to the magnetic field, causing additional trauma. Small case series in people have demonstrated no adverse effects when patients with bullets located within the spinal canal were imaged. However, most of these patients were already completely paralyzed, and metallic fragments within the spinal column should still be considered a relative contraindication for MRI.95 The stability of a vertebral fracture or luxation may be difficult to assess on plain radiography. Obviously displaced fractures are assumed to be unstable; however, minimally displaced or nondisplaced injuries require closer evaluation. Spinal fractures can be classified according to the “three column spine” principle, which divides bony and soft tissue structures into dorsal, middle, and ventral compartments, in an attempt to predict stability of the injury and make treatment recommendations.32 This system was originally proposed for fracture classification in people and has been modified for veterinary patients (Figure 34-2).88 The dorsal compartment includes the spinous processes, vertebral laminae, articular processes, vertebral pedicles, and dorsal ligamentous complex (supraspinous ligament, interspinous ligament, joint capsule, and ligamentum flavum). The middle compartment includes the dorsal longitudinal ligament, the dorsal annulus fibrosus, and the dorsal vertebral body—essentially the floor of the vertebral canal. The ventral compartment includes the remainder of the vertebral body, the lateral and ventral aspects of the annulus fibrosus, the nucleus pulposus, and the ventral longitudinal ligament. If more than one of these compartments is compromised, the fracture is considered unstable and surgical intervention is indicated. The primary downside to this classification system is that the middle compartment is difficult to assess without CT scan. A simpler classification scheme specifically assesses three principal contributors to spinal stability: the intervertebral disc, the vertebral body, and the articular processes.86 The vertebral body and articular processes can be evaluated on plain radiographs, although judging articular process integrity may require oblique radiographic views. Failure of the intervertebral disc may appear as a narrowing of the affected disc space on plain radiographs. Evaluation of the disc is imperative; it is the single most important contributor to rotational stability of the thoracolumbar vertebral motion unit.87 Failure of the disc also contributes significantly to instability in lateral bending.81 In the case of a compromised disc, an intact vertebral body provides some buttress stabilization for the spine in dorsoventral bending. Fracture of the vertebral body alone may destabilize the spine in all modes of bending and rotation.81 Even with intact disc and articular processes, vertebral body fractures are very unstable. In contrast, fractures of the articular processes, even bilaterally, can be relatively stable. Although they can result in rotational instability, articular process fractures are thought to be much less unstable than failure of the disc or vertebral body.81 Injuries with failure of more than one of these three components—intervertebral disc, vertebral body, or articular process—should be considered very unstable, regardless of the degree of displacement seen on imaging. The ideal stabilization technique for any particular injury should be based on the damaged structures and the forces acting on those specific sites. Often, this is a combination of dorsoventral and lateral bending, torsion, shear, or axial loading. However, true determination of fracture stability can be difficult, regardless of which method of assessment is used. Further, estimates of the spinal instability caused by specific fracture types are based on experimental models and may not completely reflect the clinical situation. The magnitude and vector of each force exerted on the spine by various movements such as walking, rising from lying down, or being lifted is unknown, making the required strength of fixation difficult to estimate.108 When instability of a fracture cannot be determined with certainty, it must be assumed that the fracture is unstable with respect to each of these forces, and the most comprehensive stabilization technique should be used. Initial treatment of the spinal fracture patient is aimed at management of life-threatening injuries and hemodynamic stabilization of the patient, as discussed previously. Subsequent treatment of the spinal fracture or luxation typically consists of a combination of medical and surgical therapies. Decisions should be based primarily on the neurologic status of the patient and the biomechanical and compressive characteristics of the fracture or luxation. A recommended treatment algorithm is shown in Figure 34-3. The hope for a true cure for spinal cord injury rests with regenerative therapies designed to functionally restore damaged spinal cord tissue. Although many promising strategies are currently under investigation, at this time the most realistic goal for treatment is to minimize secondary spinal cord injury and worsening of spinal cord damage after the primary injury. The relative contribution of primary and secondary injury to overall spinal cord damage is difficult to determine and varies with the type of injury and the timing of assessment. Some studies estimate that secondary injury may be responsible for as little as 10% of the overall pathology of spinal cord injury.118a Considering that locomotion requires as few as 5% to 10% of intact long tract fibers, this seemingly small contribution to injury becomes more important.8,16 Any effort to prevent secondary spinal cord injury may have a significant effect on patient outcome. The importance of maintaining spinal cord perfusion for spinal trauma patients cannot be overemphasized. Hypoxia and ischemia can significantly worsen spinal cord damage, and maintenance of normal arterial oxygenation and blood pressure is essential for minimizing secondary spinal cord injury.53 Systemic blood pressure can be maintained via combinations of intravenous crystalloid or colloid fluid therapy, blood transfusions, and vasopressors. The goal of these therapies should be maintenance of normotension, as hypertension provides no additional benefit and may in fact worsen hemorrhage or edema.74 Cardiovascular support should be maintained from initial patient stabilization through anesthesia, imaging, surgery, and recovery. It is common for spinal fracture patients to undergo prolonged anesthesia for diagnosis and treatment soon after their injury. Any steps that can be taken to minimize anesthesia time and potential anesthesia-associated hypotension may minimize secondary spinal cord injury and benefit the patient. Glucocorticoids have received significant attention as possible modulators of secondary spinal cord injury. Their use remains controversial, and their exact mechanism of action is unclear. Acute spinal cord trauma results in decreased blood flow to neural tissues. Reperfusion results in liberation of large numbers of oxygen-derived free radicals. These free radicals cause destruction of neuronal and glial cell membranes via lipid peroxidation—a major component of secondary spinal cord injury. It is believed that the primary protective effects of glucocorticoids are due to their antioxidant properties. They mitigate the damage caused by oxygen-derived free radicals by scavenging lipid peroxides in cell membranes. Methylprednisolone sodium succinate is the only neuroprotective agent that has demonstrated efficacy in controlled multicenter clinical trials in human beings. It has been recommended for the treatment of acute spinal cord injury based on the findings of the National Acute Spinal Cord Injury Studies (NASCIS-2 and NASCIS-3).19–22 NASCIS-2 demonstrated significant positive effects of methylprednisolone sodium succinate on sensory and motor function when given within 8 hours of injury and compared with placebo at 6 weeks, 6 months, and 1 year after injury. The largest effect was seen in improvement in motor function at 1 year after injury. Subjects’ motor function was graded on a scale of 0 to 70, with 0 representing no function and 70 representing normal function. This score is a summation of motor scores for multiple muscle groups over the right side of the body. Patients receiving placebo had a mean admitting score of 23.8, which increased by an average of 9.1 to 32.9 points. Patients receiving methylprednisolone sodium succinate had a mean admitting score of 21.1, which increased by an average of 14.8 to 35.9 points. The difference between 32.9 and 35.9 was shown to be statistically significant. It is important to note that patients whose treatment began after 8 hours post injury experienced worse outcomes than those treated with placebo. Based on this data, the recommendation was made to administer methylprednisolone sodium succinate within 8 hours of spinal cord injury.20 In the 20 years since NASCIS-2, the study has been the subject of major criticism. Other authors have found fault with the study’s post hoc alteration of evaluation criteria, lack of reporting of bilateral motor scores, exclusion of up to 70% of subjects from data analysis, problems with the placebo group, and lack of standardization of medical and surgical treatment regimens. Perhaps the most important criticism of NASCIS-2 is that no evaluation was performed to determine whether the statistically significant differences in sensory and motor scores that were reported represented clinically meaningful improvements in patients’ quality of life.80 Other authors have been unable to reproduce results of NASCIS-2 and -3, and methylprednisolone sodium succinate has yet to be recommended for use in acute spinal cord injury, according to the Food and Drug Administration. Recent evidence-based reviews conclude that evidence is insufficient to support the use of methylprednisolone as a standard treatment in acute spinal cord injury.80 Recent position statements put forth by respected physician medical societies in the United States and Canada have recommended the use of methylprednisolone sodium succinate as a treatment option for people, but have not labeled it as a standard of care or a guideline.42,52 According to a recent survey of North American spine surgeons who prescribe methylprednisolone sodium succinate for spinal cord trauma, fear of medicolegal issues is their most common justification; a minority of prescribing physicians believe in improved clinical outcomes with use of methylprednisolone sodium succinate.37 Recommendations for methylprednisolone sodium succinate use in veterinary patients are based largely on the results of NASCIS-2 and -3. No studies have demonstrated any benefit of methylprednisolone sodium succinate in naturally occurring spinal cord injury in animals. A variety of experimental studies of spinal cord trauma have demonstrated a positive impact of methylprednisolone sodium succinate on outcome; however, a larger number have failed to show any effect.2 Administration of methylprednisolone sodium succinate in dogs has been associated with a high incidence of gastrointestinal adverse effects, such as diarrhea, melena, and occult gastric hemorrhage.31,44,77 These can lead to bacteremia and gastrointestinal perforation.50,101 There exists the potential for adverse effects such as pneumonia, immunosuppression, and sepsis, which have been documented in human patients.42 Adverse effects of methylprednisolone sodium succinate (or any glucocorticoid) may be more likely to occur when it is administered in conjunction with other glucocorticoids or nonsteroidal antiinflammatory drugs. Methylprednisolone sodium succinate is contraindicated in these cases. In general, based on the scientific evidence, at best methylprednisolone sodium succinate should be considered a treatment option with no proven benefit and with the possibility of causing harmful adverse effects. The use of high-dose dexamethasone in spinal trauma has been investigated. Clinical studies have not shown a beneficial effect of dexamethasone on outcome,51,65 and a recent study showed an increased risk of adverse effects such as diarrhea and urinary tract infection.65 Its use cannot be recommended at this time.74 Significant research is being conducted to investigate new therapies for acute spinal cord injury, primarily focusing on neuroprotection and neural tissue transplantation or regeneration. Among treatments that hold promise are protein kinase and metalloproteinase inhibitors, glial cell and stem cell transplantation, and the use of electrical field gradients to influence and guide nerve regrowth. Some of these are nearing clinical trial status, but none have been proven effective in the treatment of acute spinal cord injury.53,74,78 Many spinal fractures are managed with a combination of surgical and nonsurgical methods. Cage rest is recommended for all spinal fracture patients, whether or not surgical stabilization has been performed. Usually 4 to 6 weeks of rest is prescribed for postoperative patients, to allow healing of soft tissue structures and to minimize early loading of spinal instrumentation. For nonsurgically managed cases, treatment typically consists of cage rest with or without external splint placement. Nonsurgical treatment avoids complications associated with anesthesia, spinal manipulation, and surgical implants. Hospitalization times may be decreased as compared with surgical treatment.84 Use of external splints may or may not be less expensive than surgical intervention, if costs of multiple sedations, bandage changes, and treatment of complications are included in the estimate.84,97 Although external splints may provide support for the injured spine, they have minimal ability to realign the spinal column. Nonetheless, several studies report good outcomes for patients managed with nonsurgical treatment alone.28,84,46,23,41 However, objective evaluation of surgical versus nonsurgical treatment is impossible based on current data. No randomized study has directly compared surgical and nonsurgical groups, and inherent biases of patient selection make retrospective studies unsuitable for this comparison. For these reasons, indications for nonsurgical management are not entirely clear. Some patients with minimal deficits and stable fractures can be managed successfully with cage rest alone. Lesions in the lumbosacral spine may be most forgiving, as any instability or compression caudal to L6 affects only the nerve roots, not the spinal cord. Patients with unmanageable pain or worsening neurologic status should be strongly considered for surgical intervention, or at least for external splinting. The best candidates for splinting are probably smaller animals with minimal neurologic dysfunction or at least normal sensation, an intact ventral buttress, and lack of concurrent thoracic, abdominal, or pelvic injuries.76 Larger dogs with more severe neurologic deficits are reported to achieve functional recovery with conservative management, even if some may not consider this to be the standard of care.28,46,23 This suggests that dogs with intact nociception whose discomfort can be managed and whose owners decline surgical treatment should not be denied the possible benefit of nonsurgical treatment with external splinting. Intractable patients or those with noncompliant owners are poor candidates for external splints. Some dogs become more agitated because of the discomfort of a splint and are poor candidates.5 Cats do not typically tolerate splints.28 External splints require intensive management to avoid complications such as decubital ulcers, urine scalding, or slipping of the splint. A displaced splint can act as a fulcrum or pendulum, worsening spinal column alignment and resulting in more damage than benefit. The goal of splinting is to immobilize the spine cranial and caudal to the site of injury. For lumbar injuries, the entire pelvis should be included in the splint, with care being taken not to restrict urination or defecation. Cervical injuries require extension of the splint from the level of the eyes to the midthorax. Splint application techniques are described in other texts.5,86 It has been recommended to maintain external splints for a minimum of 4 weeks, with an additional 4 weeks of cage rest76,86; however, the ideal duration of splinting is not known. In one study, nonsurgically treated patients that regained ambulation did so within 2 to 5 weeks of splint placement28; however, some dogs never regained ambulation. The time frame for optimal recovery is variable and may be longer than 1 year.84 Surgery is the most reliable way to stabilize the spinal column and is perhaps the only way to accurately align fractured or luxated spinal segments and decompress the spinal cord. Specific indications for surgery vary somewhat between authors. Some studies report that outcomes for surgically treated versus nonsurgically treated patients are equivalent.23,46,84 These comparisons are not based on randomized, controlled studies and are not reliable. Lack of high-quality, clinical, outcomes-based evidence requires that treatment recommendations are based largely on experimental, non–outcomes-based data, surgeon opinion and experience, and owner preference. We maintain that surgery is indicated for paretic animals with intact nociception, animals with worsening neurologic status, and those with unstable fractures or spinal cord compression. Surgery may also be indicated for animals lacking nociception whose owners are willing and able to provide long-term care for a paralyzed, incontinent animal. In these cases, surgical intervention is undertaken as a heroic measure or to facilitate the patient’s comfort without anticipation of a functional recovery. Clear communication between the surgeon and the owner is imperative in these cases, to ensure that accurate expectations and prognosis are understood. Many different surgical techniques have been described for spinal fracture stabilization in veterinary patients. This reflects the fact that no single method is best for every situation. Quite a few clinical retrospective studies and biomechanical studies have examined these techniques.* No randomized prospective studies have compared clinical outcomes between different techniques. Currently, choice of technique is based primarily on biomechanical studies, clinical retrospective studies, and surgeon preference. The thoracolumbar spine is the area most commonly affected by fracture-luxation in both dogs and cats.6,23,41,84 Lesions in this location may have a dramatic impact on spinal cord function because of the relatively small ratio of vertebral canal to spinal cord diameter.86 A variety of techniques exist for stabilization of thoracolumbar fractures. These include pins and polymethylmethacrylate (PMMA), locking plates, external fixators, vertebral body plates, modified segmental fixation, tension band stabilization, and spinous process plating. The most versatile of these techniques are pins and PMMA, locking plates, and external fixation. Although other techniques rely on the integrity of the ventral buttress for some of their stability, these more versatile techniques can be used in cases with compromised integrity of two or three vertebral compartments. Thoracolumbar stabilization is typically achieved via a dorsal approach. The patient is placed in sternal recumbency. Positioning the limbs symmetrically will aid in vertical positioning and in preventing tilting of the trunk. Poor positioning can confuse implant placement angles during stabilization. The caudal spine can be supported by a roll of towels or a vacuum bean bag positioner placed at the level of the pelvis. Some authors have expressed concern that significant padding below the abdomen can cause increased drainage of the abdominal cavity via the internal vertebral venous plexus and should be avoided.83 A dorsal approach is made to the spine and continued ventrally to the level of the costal fovea in the thoracic spine or the transverse processes in the lumbar spine. The fracture is identified and reduced. Reduction and temporary manual stabilization can be provided by distraction via towel clamps placed at the base of the spinous processes of the vertebrae adjacent to the fracture. Movements should be slow and deliberate. Excessive manipulation of the spinal column should be avoided. Reduction is often most easily evaluated by assessing alignment of the joint surfaces of the articular processes. Care should be taken not to over-reduce the fracture with excessive distraction. Once reduction is achieved, Kirschner wires can be placed through the articular processes and across the zygapophyseal (articular process) joints. This provides temporary stabilization until definitive fixation is applied. If the articular processes are fractured, or if a hemilaminectomy is needed for decompression, temporary stabilization can be achieved by Kirschner wires placed through adjacent vertebral bodies across the disc space.15 Pins and PMMA: Pins are placed in the vertebrae adjacent to the fracture or luxation. With luxations and many fractures, the pins can be inserted in consecutive vertebrae (Figure 34-4). For midbody or comminuted vertebral fractures, it may be necessary to span the fractured vertebra (Figure 34-5). Positive-profile threaded pins are preferred over other pin types. They provide better resistance to pull-out and breakage than smooth or negatively threaded pins. Most positive-profile threaded pins are hardened, thereby increasing bending stiffness. Their strength in bending is also characterized by the area moment of inertia and is proportional to radius to the fourth power. A small increase in pin radius causes a large increase in bending strength; the largest reasonable pin size should be used. Pins should be installed in a manner that maximizes the bone-pin interface. Increasing bone-pin contact results in greater resistance to pin pull-out and stronger overall constructs. Increasing the number of pins can also increase the strength and stiffness of the construct. The optimal number of pins or immobilized vertebrae has not been determined. Four-pin and PMMA constructs have been shown to be as stiff in extension, flexion, and rotation as intact spines in biomechanical testing.109 They require less exposure and fewer immobilized vertebrae than larger constructs, and appear to be effective in clinical use. Figure 34-4 Pins and polymethylmethacrylate (PMMA) engaging T12 and T13, used to stabilize a T12-T13 luxation.
Spinal Fractures and Luxations
Assessment and Diagnosis
Neurologic Examination
Imaging
Myelography
Computed Tomography
Magnetic Resonance Imaging
Biomechanical Considerations
Treatment
Medical
Nonsurgical
Surgical
Thoracolumbar Fractures and Luxations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Spinal Fractures and Luxations
Only gold members can continue reading. Log In or Register to continue