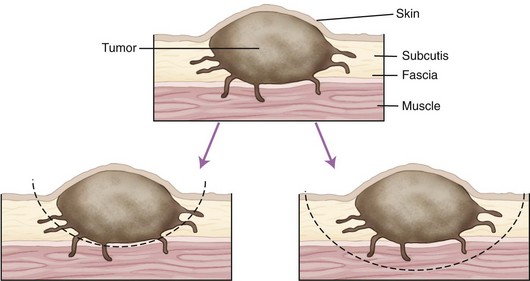
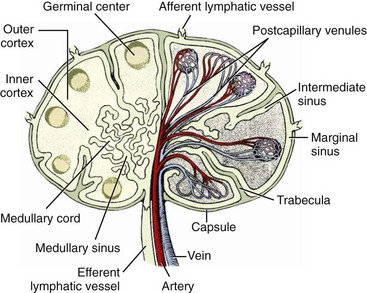
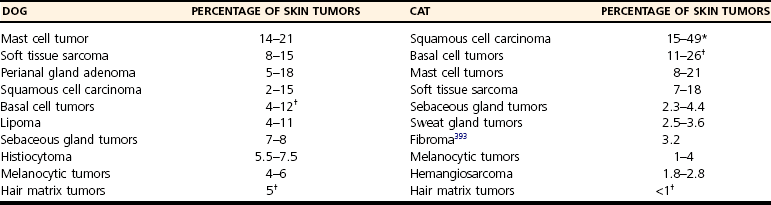
Chapter 82 Tumors of the skin and subcutis are the most common tumors in dogs and the second most common in cats (after lymphoid tumors), representing approximately one third of all tumors encountered in dogs and one fourth of all tumors in cats.624 In dogs, 20% to 40% of skin tumors are histologically malignant compared with 50% to 60% in cats.127,445,624 Surgery is the main therapeutic strategy in skin tumor management in companion animals, thus making the skin an important topic in surgical oncology. The most important factor in local tumor control is the surgical wound margin, which primarily depends on tumor type and grade. Anatomic considerations may interfere with the selected wound margins but should not be a primary determinant for extent of surgical margins. Wound margins are classified as intracapsular (tumor removed in pieces, leaving macroscopic neoplastic cells behind, often referred to as debulking), marginal (“shelling out” or removal just outside or on the [pseudo]capsule or reactive capsule; often leaving microscopic neoplasia behind), wide (tumor and capsule are not entered; normal tissue surrounds the complete excised specimen), or radical (entire compartment or structure removed)147 (Table 82-1). For wide resection, the actual margin to be taken of seemingly normal tissue surrounding the tumor depends on tumor type and grade (i.e., invasiveness) and on the type of tissue (e.g., fat versus fascia). Care should also be taken to apply surgical margins in three dimensions. For tumors with a high probability of local recurrence, the depth of dissection preferably includes at least one tissue plane away from the tumor (i.e., including underlying fascia; Figure 82-1). Table • 82-1 Because histologic type and grade are predictive for biologic behavior of many tumors, tumor biopsy is an important diagnostic tool in treatment planning.141 Tissue biopsies can be obtained using a large needle (e.g., Tru-Cut), with which multiple core biopsies should be collected. The specimens are sometimes too small for accurate histologic diagnosis, however.4 More tissue can be obtained with an incisional biopsy.141 Excisional biopsies should only be performed when adequate margins are possible. Otherwise, an incisional biopsy is preferred. Incisional biopsy tracts should be excised in continuity with the tumor because contamination of the wound bed with neoplastic cells may cause regrowth.84 Fine needle aspiration biopsy tracts are of less296 (but not zero) importance in regards to tumor spread. Contamination of needle tracts has been described for (transitional cell) carcinomas in dogs.433 Knowledge of open wound management, skin grafting, and reconstructive techniques452 will assist in more aggressive tumor resections, securing a better prognosis for the animal. Aggressive resection that requires open wound management and second intention healing is always preferred over less aggressive wound closure with a potential of leaving residual tumor tissue. Careful and correct tissue handling must be applied to avoid spreading of tumor cells,191 and manipulation of the tumor during surgery must be avoided when possible. Preferably, normal tissue along the tumor side of the resection is manipulated with retaining tissue forceps (e.g., Allison tissue forceps) or stay sutures, avoiding contamination of the surgeon’s gloves and other surgical instruments. Early ligation of larger, tumor-associated blood vessels will decrease the risk of tumor cells, freed up by manipulation of the tumor, from entering the circulation.652 If the tumor bed was incised during surgery, instruments and gloves should be changed before closure of overlying, normal tissues. However, radical recut of the wound bed should be considered, aiming at complete excision of all tumor tissue. Copious lavage of the wound bed can also help to mechanically remove exfoliated tumor cells. If more than one malignant mass is to be removed or tumor removal is combined with another surgical procedure, separate surgical instrument sets should be used to prevent contamination. Procedures involving benign lesions should be performed before removal of malignant ones. For definitive diagnosis and prognosis, resected tumors should always be submitted for examination to a certified veterinary pathologist. The complete specimen is preferably sent in after fixation in 5% to 10% formaldehyde; large specimens should be incised to allow proper fixation. Areas of special interest should be marked for the pathologist with sutures, India ink, or specially developed dyes.541 Pathologic results can determine additional treatment such as chemotherapy or radiation therapy. In case of incomplete excision of a malignant tumor, follow-up surgery (recut) may be considered; the previous wound bed (including the scar) should be completely excised with new fresh wound margins of 2 to 3 cm of normal tissue. Chemotherapy can affect wound healing to some extent, although few clinically relevant complications occur in patients receiving perioperative chemotherapy. The effect depends on tissue type and agents used.251,453 Intestinal tissues seem more sensitive309 and carry a risk of severe postoperative complications. In general, it is advised to start chemotherapy 7 to 10 days after surgery.155,453,652 Studies of perioperative chemotherapy in women with breast cancer68,545 or ovarian cancer,300 however, showed no significant effect on wound healing of skin and subcutaneous tissues. Furthermore, postoperatively activated physiologic wound healing pathways may play a role in tumor metastasis; thus, perioperative chemotherapy might improve long-term survival in tumor patients.225 The best moment to start adjuvant chemotherapy is therefore arbitrary until further research provides more detailed information. Use of steroids as part of a chemotherapeutical protocol follows the same guidelines.453 Radiation therapy induces dose-dependent injury to tissues,614 and subsequent tissue damage is more permanent than that of chemotherapy. Effects on surgical wound healing are most distinct in the acute inflammatory phase and the following proliferative phase211 and are less severe during granulation and remodeling.614 The first 5 to 8 days after surgery are most critical for healing;632 adjuvant radiation therapy is therefore advised to start at 1 to 3 weeks after surgery.312,632,652 Again, the interval between surgery and radiation poses a theoretical dilemma about physiologic wound healing versus tumor behavior.225,632 In cats, for example, an increased time interval between surgery and radiation for soft tissue sarcoma significantly decreased disease-free interval and survival time.93 If preoperative radiation therapy is given, the general advice is to delay surgery until clearance of acute radiation effects, generally for a period of 3 to 4 weeks.614,632,652 Tumor-related causes of complicated wound healing include residual neoplastic tissue infiltrating and disrupting normal tissue environment, tumor-related cytokines and bioactive substances (e.g., mast cell degranulation and fibroblast suppressor factor associated with mast cell tumors), cancer cachexia, and other paraneoplastic syndromes.97,155,364 Existing neovascularization requires adequate hemostasis to avoid excessive bleeding and formation of postoperative hematomas. Use of electrosurgery and laser can decrease bleeding and improve hemostasis. Seroma formation can usually be prevented with proper surgical techniques (i.e., correct wound closure, preserving blood supply, avoiding dead space, gentle tissue handling, and use of draining techniques if indicated). Apart from immunosuppression, tumor-related factors and decreased immunity induced by adjuvant therapies may affect wound healing and subsequently render the wound more susceptible to infection. Prophylactic use of antibiotics, however, must be based on individual criteria and should not be used as a standard therapy. Skin tumors are histologically classified according to tissue of origin (mesenchymal, epithelial, melanocytic, or round cell) and the individual tumor cell type. Further classification describes the degree of malignancy based on several histologic parameters. Clinical staging of skin tumors uses the tumor–node–metastasis (TNM) system (Table 82-2) developed by the World Health Organization (WHO).442 TNM classifies a tumor by its size and invasiveness (T), involvement of regional lymph nodes (N), and presence of distant metastases (M). Furthermore, tumor location on the skin can be of prognostic importance, and tumors in dogs can have different biologic behavior compared with tumors of the same type in cats. Table • 82-2 World Health Organization Tumor–Node–Metastasis Classification for Canine and Feline Tumors of Epidermal or Dermal Origin (Excluding Lymphoma, Mastocytoma, and Mammary Tumors)442 A proper patient workup includes signalment and a thorough anamnesis. Age, breed, and sex can guide the clinician toward an initial differential diagnosis of most common malignancies. Anamnesis should focus on signs of chronic or acute general health problems (changes in body weight, eating, and drinking; exercise intolerance; coughing), and signs of possible paraneoplastic syndromes (gastric problems associated with mast cell tumors, polyuria and polydipsia with hypercalcemia from anal sac carcinoma). In addition to disease history, clinical history (prior treatments and their effect) is of importance. Information concerning the duration of the lesion, speed of growth, inflammatory signs, pruritus, and change in appearance over time should be gathered. A complete general physical examination is performed, and the tumor is examined in detail. Tumor parameters are retrieved, including size (three-dimensional caliper measurements), anatomic location, consistency, color, presence or absence of fixation to surrounding tissues, ulceration of overlying skin, and signs of inflammation. For medical recording, it is valuable to also take pictures. Meticulous physical investigation of draining lymph nodes is a standard part of the procedure. Lymphadenopathy may result from metastasis (firm, irregular, occasionally fixed to surrounding tissues) or from reactivity to tumor factors, infection, or inflammation (especially with ulcerated or inflamed tumors).183 An additional workup of the patient is mandated by other factors such as the clinical status, age, and tumor type and often includes routine blood work and diagnostic imaging techniques. All skin and subcutaneous masses should be investigated cytologically by fine needle aspiration biopsy. Cytology of skin masses has a high predictive value of diagnosing neoplasia, and the accuracy of cytologic diagnosis compared with histopathology is estimated to be more than 90%.79,184 Most tumors can be diagnosed cytologically. However, definitive diagnosis of tumor type and grade (malignancy) should be confirmed through histology. Furthermore, some tumors are difficult to diagnose cytologically, and side effects such as inflammation can be more prominent than the tumor itself.549 If the result of fine needle aspiration biopsy does not comply with clinical diagnosis, tissue biopsy is often warranted. The route of metastasis varies between tumor types. Whereas epithelial neoplasia tends to metastasize via lymph drainage, mesenchymal neoplasia spreads commonly through hematogenous routes. This is merely a general rule of thumb,316 and any enlarged draining lymph node requires further investigation before definitive treatment planning of the patient. Furthermore, tumor cells that formerly were thought to spread via the lymphatic system were observed to also travel through blood vessels, and experiments in rodents have shown that tumor emboli can pass through lymph nodes to appear in efferent lymph vessels and the thoracic duct. Recent research illustrates that various tumor types have organ specificity for metastases. This organ specificity may be caused by surface adhesion molecules, detected on both tumor cells and tissues where metastases develop.63,467 Fine needle aspiration cytology or biopsy can be very sensitive to diagnose lymph node metastasis in cases of a uniform metastases pattern, in contrast to manual palpation, which has much lower sensitivity.316 One may therefore consider sampling all palpable regional lymph nodes. A “positive” node often influences the prognosis72,248,282,418 and warrants further investigation for distant metastasis. For skin tumors, complete excision of the lesion with local curative intent is the most commonly applied therapeutic strategy. Excisional biopsy can be diagnostic and curative in selected cases of relatively small, slowly growing, freely movable lesions that are easily accessible for surgery; show no signs of inflammation; and are not painful or pruritic. Any doubt about the malignancy of a lesion necessitates either wide margins (2 to 3 cm around macroscopically visible tumor margin is generally considered for locally invasive tumors; see section on mast cell tumors and soft tissue sarcoma) or further diagnostic workup. Large masses, uncertainty of margins on palpation, close proximity to vital structures, fixation to surrounding tissues, suspicious clinical findings, or cytology or biopsy results will dictate further diagnostic imaging techniques, such as ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI), to establish better insight in tumor margins and invasiveness.215,371,499 Depending on the tumor type and expected biologic behavior, draining lymph node status, general health status, and tumor presentation (e.g., invasive, ulceration), further workup and investigation of distant metastasis may be indicated before definitive treatment. If available and if financial means permit, screening for thoracic metastasis is preferably done by CT scan, which is more sensitive for detection of small nodules compared with standard radiography (1 mm compared with 7 to 9 mm diameter, respectively).422 The lymphatic system has two major functions: transport and immune response. As part of the cardiovascular system, the lymphatic system collects and transports lymph from tissues and organs; transports lipids from the intestines and liver; and transports cellular debris, metabolic waste products, and fluid excesses (preventing edema formation) from local sites back to the systemic circulation. As part of the host immune defense system, it acts to detect infectious agents and alien particles and start an immune response. This protective role is reflected in the distribution of lymphatic vasculature and lymphoid tissue in organs that come into direct contact with the external environment, such as the skin, gastrointestinal tract, and lungs. The lymphatic vasculature consists of initial lymphatics, or lymphatic precollectors, which coalesce into lymphatic ducts, which then drain into the lymph nodes.592 The initial lymphatics are present as blind-end sinuses, formed by single layers of lymphatic endothelial cells. Initial lymphatics range in diameter from 10 to 60 µm, significantly larger than the diameter of arteriovenous capillaries (8 µm). Lymphatic endothelial cells rest on a discontinuous basement membrane that is attached to surrounding connective tissue by anchoring filaments. There are no tight junctions between the cells, and interendothelial openings permit extracellular fluid, macromolecules, and cells to drain directly into the lumina of the initial lymphatics through the porous basement membrane. Endothelial cells are arranged in an overlapping structure that can provide a one-way valve system for fluid movement. Interendothelial junctions open during fluid inflow from the interstitium because of stretching of the lymphatic endothelium or by edema. In theory, reflux of lymphatic fluid into the interstitium is prevented by reclosure of the endothelial clefts. The initial lymphatics are connected with the deeper lymphatics in the dermis. There, lymph is transported centrally through collecting ducts and, subsequently, to the lymph nodes. The collecting ducts have thick walls (0.50 to 0.75 mm in diameter) and contain a more distinct intima, media, and adventitia and a thin layer of smooth muscle.166,592 They are innervated by sympathetic and parasympathetic nerve systems and have an intrinsic nervous plexus.154,166 Lymph flow in lymphatics is believed to be active, by constriction of smooth muscle in the vessel wall, and passive, through pressure from outside (skeletal muscle movement, artery pulsations), with lymphatic valves preventing retrograde flow.154 Eventually, lymph enters the systemic circulation through the thoracic duct. All lymph vessels pass through at least one node. Lymph nodes are found in the small fat deposits at flexor angles of joints, in mediastinum and mesentery, and in the bifurcation of many larger blood vessels. Dogs and cats usually have one or two large nodes at each nodal station.154 Each node consists of a capsule containing elastic and smooth muscle fibers and an internal framework consisting of septa and trabeculae. There is a convex surface and a small flat or concave area, the hilus. Internally, the node contains a cortex and medulla. Lymph vessels enter the lymph node as afferent lymph vessels. They break up into many small vessels before perforating the capsule of the node and connect to the lymph sinus system inside the node. These subcapsular, cortical, and medullary lymph spaces or sinuses unite, and the lymph leaves the node at the hilus through one or more efferent lymph vessels (Figure 82-2).154,498 The cortex consists primarily of B-lymphocytes arranged into follicles, with germinal centers (B-lymphocytes and plasma cells) surrounded by a rim of T-lymphocytes when the node is challenged antigenically.519 Between cortex and medulla lies the paracortex, which is composed of T-cells and antigen-presenting cells (macrophages). The medulla is composed of cords of lymphocytes, macrophages, and plasma cells. Between these medullary cords are sinuses lined with discontinuous endothelium, along with macrophages and reticular cells to filter and phagocytose foreign material.498 Lymph nodes drain lymph from a certain area of the skin or organ, filtering out foreign material and eliciting a primary immune response. Evidence of metastasis in local lymph nodes is an important indicator of systemic metastasis and predictive for prognosis of several cancers in small animals, including canine mammary tumors,307 mast cell tumors, small intestinal tumors,101 and canine primary lung tumors.375 For humans with melanoma, the status of draining lymph nodes is the most accurate prognostic indicator of systemic spread.21 However, apart from being an important prognostic factor, the role of positive nodes in tumor metastasis is unclear, and how to deal with tumor-positive local lymph nodes remains a controversial subject in both human and veterinary medicine. The classic theory that the lymph node blocks tumor cells and represents the first location of metastasis before systemic spread carries no solid scientific basis.182,467 This is further denoted by the fact that many large human clinical trials failed to show an improved survival after elective lymphadenectomy (removal of local lymph nodes irrespective of tumor metastasis) compared with lymphadenectomy of only tumor-positive nodes or delayed lymphadenectomy at the time of tumor recurrence in local lymph nodes.182,467 Furthermore, tumor sites are commonly drained by more than one set of local lymph nodes, and metastasis can occur in several differently located nodes,244 making elective lymphadenectomy a serious and invasive procedure. Nowadays, local lymph node staging in humans is based on biopsy of the sentinel draining lymph node.506 This sentinel node is made visible using blue dye or a low dose of radionuclide injected in the tumor. The first draining node is detected visually or by hand-held gamma camera and removed for histology (frozen section). If the sentinel node is negative for metastasis, dissection of subsequent lymph nodes may be suspended. Although a definitive role for lymphadenectomy remains unclear, lymph node metastases may act as a new source for further spread of tumor emboli, and lymphadenectomy could possibly slow down the rate of metastasis. Likewise, lymphadenectomy may reduce clinical signs of paraneoplastic disease and palliate symptoms caused by enlarged lymph node dimensions, such as seen with canine anal sac adenocarcinoma (see Canine Anal Sac Adenocarcinoma). Lymphadenectomy may be part of a tumor-debulking procedure (to increase local control) and may be combined with adjuvant therapies, which usually have better effectiveness in the absence of macroscopic disease. Lymphadenectomy of apparently normal nodes (on fine needle aspiration biopsy) is potentially harmful (see section on lymphedema) and possibly interferes with an important immunologic host response against the tumor and therefore should not be performed.467 The draining function of the lymphatic system makes it susceptible to secondary inflammation from diseases, particularly of the skin, mucous membranes, gastrointestinal tract, and subcutaneous tissues. Lymphangitis (inflammation of lymph vessels) is usually caused by infectious agents, including (myco)bacteria, fungi, and parasites.301,482 Introduction of the infectious agent can be minor trauma or a wound. A parasitic infection by Brugia spp. (filariasis) can cause granulomatous lymphangitis and lymphadenitis in cats and dogs, which may play a role as reservoir for human filariasis (Brugia malayi)83,360 in tropical areas (see also next section on lymphedema). Affected limbs are locally swollen and painful, and lameness can result. Pyrexia, anorexia, and depression can occur, and leukocytosis is often present with acute, severe lymphangitis. An erythematous linear area (lymph vessel) may occur, connecting the primary site of infection with the associated lymph node.183,482 Lymphangitis can also be nodular,301 such as seen with sporotrichosis. Impairment of lymph drainage may cause swelling of a complete limb (see also next section on lymphedema). Persistence of inflammatory edema results in mesenchymal cell proliferation, which may cause irreversible thickening of skin and subcutis (Figure 82-3). Lymphangitis is usually diagnosed on the basis of clinical presentation. Further diagnostic workup, such as cytology of exudate, bacterial culture and sensitivity testing, and histopathology, may aid in identifying an underlying cause. If draining tracts are present, ultrasonography or contrast radiography can be performed to investigate the presence of a foreign body and the extent of tissue alterations. Conservative therapy using moist, warm, local compresses or soaks is aimed at reducing swelling and promoting drainage. If an infectious agent is diagnosed or suspected, systemic antimicrobial therapy is indicated. Initial empirical therapy can consist of broad-spectrum antibiotics against aerobic, Gram positive bacteria. Bacterial culture and sensitivity testing should be performed if acute lymphangitis fails to respond to treatment and in chronic cases. Antibiotic treatment is continued at least 1 week after resolution of clinical signs. Mycotic infections require longer antifungal therapy. Surgical exploration may be indicated for fistulous tracts or abscesses to remove a potential foreign body or chronic lesions.167,223 Interstitial edema is caused by a fluid imbalance between net capillary filtration and lymphatic return of interstitial fluid. This imbalance can be secondary to too rapid formation of lymph fluid (high lymphatic load) caused by venous hypertension (congestive heart failure), portal hypertension, venous obstruction, arteriovenous fistula, decreased plasma oncotic pressure (hypoproteinemia), or increased vascular permeability with vasculitis. Reduced lymphatic transport capacity may also cause interstitial edema, called lymphedema.166,634 With lymphatic stasis, (macromolecular) proteins and cellular metabolites accumulate in the extracellular space. Because of increasing tissue colloid osmotic pressure, more water accumulates, and interstitial hydraulic pressure increases. Lymph stasis may cause dilatation of lymphatic vessels with secondary valve insufficiency and decreased contractility. In most (human) patients, there is an increase in collagen deposition with adipose and connective tissue overgrowth in the edematous skin and subcutaneous tissues. Histologic findings in chronic lymphedema include thickening of the basement membrane of lymphatic vessels and increased numbers of mononuclear inflammatory cells (mainly macrophages) and fibroblasts, producing increased amounts of collagen. Ultimately, these processes lead to progressive subcutaneous fibrosis.73,106,166,495,592 Primary Lymphedema: Primary lymphedema in humans is a heterogeneous group of disorders, many of which show an autosomal dominant pattern of inheritance.494,634 They are usually classified based on the time of onset of clinical signs such as congenital hereditary lymphedema, or Milroy disease (0 to 2 years of age); familial lymphedema praecox, or Meige disease (puberty); and lymphedema tarda (onset after 35 years of age). Recent genetic studies have linked Milroy disease to an inactivation mutation of the vascular endothelial growth factor receptor 3 (VEGF3) tyrosine kinase signaling pathway found in lymphatic vessels.283,658 In companion animals, primary lymphedema is a rare disease mostly seen in dogs. The veterinary literature of lymphedema consists of case reports of individuals or small numbers of animals affected mostly with primary congenital lymphedema and a young age of onset of clinical signs (usually at birth).166 A lethal, congenital form of generalized lymphedema was reported in bulldogs; the authors suspected a genetic cause because of the relatively high incidence in four breeding kennels.310 Congenital lymphedema with autosomally dominant inheritance was described for a poodle and her offspring. Clinical signs varied from generalized lethal edema to mild effects of one or two hindlimbs that occasionally disappeared within the first 3 months of age.451 Congenital lymphedema has been described in cats.267 Known underlying abnormalities in primary lymphedema are hypoplasia, aplasia, or hyperplasia of distal lymphatics; lymphatic valve incompetence; and hypoplastic or fibrotic lymph nodes.166,495,592 Lymph node aplasia of the affected limb has been described in dogs with primary lymphedema.109,322,451 A rare complication of chronic lymphedema in humans is the development of cutaneous malignant tumors, such as lymphangiosarcoma.510 Lymphangiosarcoma is very rare in companion animals.646 It was reported in a Bouvier des Flandres with primary lymphedema.635 Secondary Lymphedema: Known causes of lymphatic impairment in secondary lymphedema are neoplasia, trauma, surgery, radiation therapy, parasitic infection, and chronic lymphangitis.166,494 The most common cause of secondary lymphedema in humans is infection with lymphatic filariasis (Wuchereria bancrofti and B. malayi), affecting an estimated 90 to 120 million humans in tropical countries.446,634 Adult filarial worms obstruct peripheral lymphatics, disrupting lymphatic transport. Domestic cats may be a reservoir for human infection with B. malayi. Cats usually do not develop lymphedema as seen in humans but do develop granulomatous lymphangitis and lymphadenitis.83,360 In the Western world, secondary lymphedema in humans is most commonly caused by neoplasia or its therapies, breast cancer in particular. Lymphedema of the arm is reported 24% to 49% after mastectomy and 4% to 28% after lumpectomy. Radiation and axillary lymph node dissection increase the risk of edema formation.634 Use of sentinel lymph node biopsy (sparing axillary lymph nodes if the sentinel node is negative for tumor cells) has decreased the occurrence of postoperative lymphedema compared with traditional axillary node dissection.188,506,634 Attempts to use dogs as a model for human peripheral lymphedema have shown that lymphedema does not occur unless major disruption of the lymphatic system is achieved. Alternative lymph routes can be formed by collateral lymphatics, dermal backflow and rerouting of lymph, or local lymphaticovenous shunting.86,586 Apart from experiments using canine lymphedema models for postsurgery86 and filarial lymphedema in humans,439 specific veterinary literature on secondary lymphedema in companion animals is limited. Two dogs were reported with secondary lymphedema of the hindlimbs caused by neoplasia.280 Diagnosis of lymphedema is usually made on the basis of clinical signs and history. Primary and secondary lymphedema are difficult to distinguish because an acquired disorder may be superimposed on an already impaired lymphatic system, and the onset of edema can occur after minor trauma. Taking a thorough patient history is important and should include information concerning trauma, former surgery, age of onset, progression of disease, and signs of infection. Lymphedema usually has a gradual onset in the distal limb that progresses proximally and is initially pitting in nature, not painful, and not warm.165 As fibrosis increases, pitting may not occur. Important physical findings include the extent of edema formation and evidence of underlying disease. High lymphatic load (inflammation, hypoproteinemia, cardiac dysfunction, venous obstruction, arteriovenous fistula) must be ruled out. Venous flow from the affected area can be evaluated by Doppler ultrasonography or contrast venography. Lymphatic impairment can be investigated by direct contrast lymphangiography or (indirect) lymphoscintigraphy (the current standard in human medicine).165,280,494,592 CT and MRI are also used.634 Criteria used for diagnosis of lymphatic dysfunction include delayed, asymmetric, or absent visualization of regional lymph nodes; asymmetric visualization of lymphatic channels; collateral lymphatic channels; interrupted vascular structures; and visualization of lymph nodes of the deep lymphatic system. The presence of “dermal backflow” is abnormal and is generally interpreted to represent extravasation of lymph from the vasculature into the interstitium as a consequence of lymphatic hypertension.494,587 A simple indirect lymphography method using the patent bleu violet dye absorption test has been used in the diagnosis of primary lymphedema in dogs424 and a cat.267 A sterile 5% patent bleu violet dye solution is injected subcutaneously between the digits of the affected limb. Local, diffuse distribution of dye demonstrates absence of intact lymphatic transport.267 Lymphoscintigraphy was successfully used in the diagnosis of two dogs with secondary lymphedema of the hindlimbs caused by neoplasia280 and in one dog with primary lymphedema.635 Causes of secondary lymphedema should be further investigated (e.g., cytology or biopsy of masses and abnormal tissues or lymph nodes) when applicable. Treatment options for lymphedema are aimed at improving lymphatic transport. Today, the main treatment in human lymphedema is “complex decongestive therapy,” which consists of specialized physiotherapy (manual lymphatic drainage) combined with exercise, skin care, and local compression with bandages and elastic garments.494 Only limited cases are reported of dogs with lymphedema in which clinical signs improved with pressure bandage treatment.322,593 Pharmacologic treatment options are sparse, and their efficacy is controversial. Benzopyrones (coumarin) have been used in the therapy of lymphedema for their theoretical effect on cutaneous macrophages, leading to increased local proteolysis. Although randomized, placebo-controlled studies in humans have shown significant effects on lymphedema with use of benzopyrones,74 positive effects of these drugs and study results are not universally acknowledged.18 Furthermore, long-term use is limited because of documented hepatotoxicity when given systemically.336 Their effects have not been reported in veterinary medicine. Diuretics have no role in lymphedema management because edema is a result of increased interstitial oncotic pressure produced by macromolecules rather than primary retention of water and electrolytes. Multiple surgical treatments have been proposed and applied to humans in whom response to conservative treatment is insufficient, most likely because of subcutaneous adipose tissue hypertrophy and fibrosis.193,495,634 No surgical treatment has been consistently successful in treating patients with lymphedema. Procedures aimed at improvement of lymph drainage include microsurgical creation of anastomoses between lymphatic vessels and veins, between lymph nodes and veins, and between distal and proximal lymphatics. Microsurgery has lately shown promising results, especially in earlystage lymphedema.65 Other surgical techniques to improve lymphatic drainage include treatment with transfer of an omental pedicle flap,199 interposition of vascular pedicle and axial pattern flaps,447,477 or free muscle flap.91 Radical excision of redundant subcutaneous lymphedematous tissue can reduce symptoms289 but does not improve lymph drainage and may result in skin complications.193 A recent debulking method for lymphedema using circumferential liposuction combined with compressive garments achieved 106% lymphedema reduction at 4 years of follow-up.54–56 In general, long-term use of compressive garments is always indicated in humans despite treatment method.634 The latest research on lymphedema treatment investigates lymphangiogenesis using certain growth factors.603 In veterinary medicine, few cases have been reported concerning surgical treatment of patients with lymphedema. Excision of subcutaneous tissue and fascia was performed in two dogs with significant reduction in edema.322 In other reports, one dog developed cellulitis and a nonhealing ulcer after surgical excision of edematous tissue and required limb amputation,208 and another dog had no beneficial response.189 Knowledge about malignant skin tumors is essential in surgical oncology because prognosis and treatment strategy depend on it. The growth pattern, malignancy, route of metastasis, and responsiveness to therapy (surgical as well as adjuvant chemotherapy or radiation) differ among tumor types and grades. This chapter therefore focuses on malignant tumors of the skin and subcutaneous tissues. The most common skin tumors of dogs and cats are presented in Table 82-3. Because surgical therapy of benign skin masses is alike in most cases, benign tumors and their therapy are only described if they carry specific features. In general, benign tumors can be completely excised with relatively small wound margins, incorporating the visible tumor margin or (pseudo)capsule. Table • 82-3 Most Common Skin Tumors of the Dog and Cat, Excluding Mammary and Lymphoid Tumors *Incidence of feline squamous cell carcinoma varies considerably with geographic location. †Nomenclature of basal cell tumors has been subject to changes recently, and many tumor types that used to be categorized as basal cell tumor are now categorized as specific adnexal tumors. Skin tumors are often classified by cell of origin.308 Mesenchymal tumors arise from embryonic mesoderm and include those of connective tissue, endothelial, hematopoietic, lymphoid, and muscle origin. Fibrosarcomas, hemangiopericytomas, peripheral nerve sheath tumors, myxosarcomas, liposarcomas, lipomas, hemangiosarcomas, vaccine-induced sarcomas, and melanomas are mesenchymal tumors. Cells of tumors from hematopoietic and lymphoid tissues are usually small, discrete, and round and are therefore occasionally subclassified as discrete round cell tumors based on their cytologic characteristics. Lymphoma, plasma cell tumor, histiocytoma, mast cell tumor, transmissible venereal tumor, and malignant histiocytosis are typically included in this group. Epithelial tumors can arise from endoderm, mesoderm, or ectoderm and include glandular and nonglandular tumors. Malignant epithelial tumors are termed carcinomas. Included in this group are papillomas, squamous cell carcinoma, basal cell tumors, sebaceous and sweat gland tumors, perianal adenomas and adenocarcinomas, apocrine gland adenocarcinomas, hair matrix tumors, and mammary gland tumors. Papillomas (warts, verrucae) are common benign epithelial proliferations in young and old dogs and are rare in cats. They usually are cauliflower-like in appearance and are associated with a DNA–viral cause. Papillomas of older dogs were formerly not believed to have a viral etiology; however, recent research using polymerase chain reaction (PCR) techniques has isolated canine oral papilloma virus from papillomas of the oral cavity, lips, skin, and conjunctiva44,601 and novel canine cutaneous papillomaviruses from cutaneous canine viral pigmented plaques.615,616 Papilloma virus has also been detected in papillomas of cats.589 Histomorphologically, cutaneous papilloma virus–induced lesions are classified as exophytic papilloma, inverted papilloma, and canine viral pigmented plaque.210 Papilloma infection can occur directly and indirectly. In young dogs, papillomatosis often presents as multiple papillomas on the head, mouth, eyelids, and feet. The lesions usually resolve within 3 months, and a specific therapy is not needed. In older dogs, the lesion is commonly solitary. Surgical removal (excision or cryosurgery) is only indicated when tumors cause problems because of size or location and is associated with a good prognosis.624 Squamous cell carcinoma is a malignant, locally invasive skin tumor that is common in dogs and cats. The reported prevalence, which varies considerably, is 2% to 15% of all cutaneous tumors in dogs277,411,505 and 15% to 49% in cats.210,393,505 This variation is probably because of geographic location: higher rates are seen in subtropic and tropic areas because of development associated with solar exposure.210,411 Unpigmented or lightly pigmented skin is most commonly involved, and a relationship to sunlight exposure is recognized in many cases.132,345,428 In humans, squamous cell carcinoma is highly associated with actinic keratosis, a precancerous solar-induced lesion,102 which may be considered the earliest form of squamous cell carcinoma in situ (preinvasive carcinoma that is confined to the basement membrane).210,333 An estimated 0.25% to 1.0% of actinic keratoses per year progress to invasive squamous cell carcinoma,271 and more than 80% of human squamous cell carcinomas were related to previous actinic keratosis lesions.400 Similarly, actinic keratosis and squamous cell carcinoma often coexist in dogs and cats.210 A rare form of carcinoma in situ in cats that is very rare in dogs (one case report)209 is multicentric squamous cell carcinoma in situ. It resembles Bowen disease in humans and is therefore termed Bowenoid in situ carcinoma in cats.210 These lesions usually present as multifocal, crusted plaques occurring anywhere on the body (pigmented as well as nonpigmented skin) and may contain melanin. Seventeen percent of the reported cases in cats and the one in a dog progressed to invasive squamous cell carcinoma.210 Ultraviolet light–specific mutations in the p53 tumor suppressor gene have been reported in 54% to 75% of squamous cell carcinomas in humans.98,287,420 Likewise, mutations in the p53 gene have been reported in 53% and 32% of cutaneous squamous cell carcinoma in cats and dogs, respectively6,417,602 and in 48% of Bowenoid in situ carcinoma in cats.156 Other risk factors for squamous cell carcinoma formation in humans include chronic inflammation and thermal injury.303 There is one report of a dog developing squamous cell carcinoma in a burn scar.202 Apart from the well-documented relationship to solar exposure and thermal injury, squamous cell carcinoma has been suggested to be associated with papilloma viruses in humans.5 A few studies found papilloma virus in squamous cell carcinoma lesions in dogs,535,601,660 suggesting an etiologic relation. In cats, squamous cell carcinoma and Bowenoid in situ carcinoma have recently been associated with papilloma virus in 11 of 18, 17 of 20, and 20 of 20 lesions,414–416 11 of 23 lesions,156 and 4 of 22, and 5 of 21 lesions423 using PCR and immunohistochemistry. Papilloma virus has, however, also been found in normal cat skin (in 3 of 17 control subjects) and, as with human and canine squamous cell carcinoma, definitive proof of a viral cause of squamous cell carcinoma in cats is difficult to obtain.414 The mean age of affected dogs is 8 years, and the mean age in cats is 12 years. In dogs, the most common locations of cutaneous squamous cell carcinoma are the nail bed (see also section on subungual and digital tumors), scrotum, legs, and anus. Nasal planum squamous cell carcinoma has a higher incidence in Labrador and Golden retrievers, with an overrepresentation of intact males.319 Squamous cell carcinoma on nonpigmented and lightly pigmented skin of the ventral abdomen and flank has been reported in dalmatians, beagles, whippets, and white English bull terriers.624 Other high-risk breeds are Pit Bull Terriers and harlequin Great Danes. In general, short-coated dogs with white or piebald ventral coat color are affected.210 In cats, the most common locations of cutaneous squamous cell carcinoma are the pinnae, eyelids, and nasal planum (Figure 82-4). Multiple facial lesions exist in 30% of the cases.624 White cats have a 13 times higher risk compared with cats with other coat colors.511 Siamese cats are underrepresented, probably because of their pigmented skin color.624 Squamous cell carcinoma can be proliferative or erosive. Proliferative squamous cell carcinoma usually present as plaque-like or cauliflower-like lesions of several millimeters to centimeters in diameter. Alopecia, erythema, ulceration, and crusting are often present. The erosive lesion, which is usually seen in cats, starts as a shallow crusting lesion that may develop into a deep ulcer. The initial superficial crusting lesions in cats often represent carcinoma in situ (clinical stage Tis). Histologically, squamous cell carcinoma is usually classified as well, moderately, or poorly differentiated.210 Cutaneous squamous cell carcinoma of the flank and ventral abdomen of dogs is generally locally invasive with a low metastatic potential. Multiple lesions with different grades of malignancy may, however, be present. Squamous cell carcinoma involving the facial skin of cats and dogs are reported to be locally invasive but late to metastasize.624 The tumors may become highly invasive and locally destructive, however, with treatment much more successful in Tis and T1 stages compared with more invasive lesions. In general, the cause of death or euthanasia for animals with invasive squamous cell carcinoma of the nasal planum and face is usually extensive local progression or recurrence.314,319,499 Squamous cell carcinoma of cats’ pinnae are better manageable because that location generally admits wide surgical resection.314 Surgical excision is the most common treatment used for squamous cell carcinoma. A median survival of 673 days (n = 39) was reported after nosectomy and pinnectomy for squamous cell carcinoma in cats.314 Apart from surgery, a variety of alternative treatments have been reported to be effective for squamous cell carcinoma stages Tis and T1.314,612,624 For higher stage tumors (≥T2), aggressive surgery seems to give the best prognosis.* Cryosurgery: Cryosurgery can be used for superficial, early-stage, small lesions. A study of 90 cats with nasal planum lesions reported 1- and 3-year disease-free intervals of 84% and 81%, respectively, although many required multiple treatments.90 Median survival of 682 days (n = 11) was reported by Lana et al.314 Cryosurgery is also commonly used in humans for small superficial and precancerous lesions.622 A disadvantage is that no histologic investigation of margins is possible. Cryosurgery should probably only be used for small superficial lesions of up to 5 mm in diameter.314,612 Treatment outcome is strongly related to technique, especially the applied freezing time,605 and tumor depth. Recently, 18 feline squamous cell carcinomas ranging in size from 0.3 to 2.2 cm in diameter were frozen with liquid nitrogen spray in three freeze–thaw cycles, applying a 15- to 60-second continuous freezing time. Lesions of 1 cm or larger in diameter (n = 7) were surgically debulked before cryosurgery. All lesions went into complete remission and were still in remission at a median follow-up of 420 days.116 Plesiotherapy: With strontium plesiotherapy, a 90Sr probe is held directly at the lesion. Plesiotherapy is a superficial form of radiation therapy, and the applied dose shows a rapid decrease in depth of the tissue, with less than 10% of the surface dose reaching 3 mm of depth.380 Recent studies showed long-term responses to therapy. One-, 2-, and 5-year progression-free intervals of 88%, 80%, and 49%, respectively, were found for squamous cell carcinoma (n = 35) and squamous cell carcinoma in situ (n = 14).218 Similar results are mentioned in an abstract of a study of 25 cats with early superficial lesions of less than 3 mm diameter, with 1- and 3-year control rates of 89% and 82%, respectively.627 In a recent study of 15 cats primarily with T2 lesions, 1-year survival was 77% to 85%; the population was too small for long-term survival analysis.200 Radiation Therapy: In a retrospective study of 90 cats receiving radiation therapy (40 Gy in 10 fractions) for squamous cell carcinoma of the nasal planum, an estimated 1- and 5-year progression-free survival of 85% and 56%, respectively, for stage T1 squamous cell carcinoma was calculated. Higher stage tumors showed a poor response.610 A median survival interval of 383 days was reported for 11 cats, although the exact stage (tumor dimensions) was unknown.314 In a study of 17 cats with mainly stage T2 and higher nasal plane and periocular squamous cell carcinoma, 1- and 2-year disease-free-interval rates of 66% and 40%, respectively, were found after electron-beam radiation treatment (94% complete response rate).379 These findings are comparable to 1-year control rate of 67% reported for stage T2 nasal planum squamous cell carcinoma in cats by Theon et al.610 Proton-beam treatment of 15 cats resulted in 1-year tumor control rate of 64% (93% response rate and 60% complete response) and median survival of 946 days.159 Photodynamic Therapy: Superficial squamous cell carcinoma in dogs and cats has been treated with photodynamic therapy using various photosensitizers.158,171,339,367,440 A recent study of photodynamic therapy in 55 cats with superficial squamous cell carcinoma (mainly T1) of the nasal planum using topical 5-aminolevulinic acid resulted in a 93% response rate; 85% showed a complete response, 18 cats received a second treatment after recurrence, and 45% of the cats were disease free at a median follow-up of 38 months.35 Systemic use of the photosensitizer meta-(tetrahydroxyphenyl)chlorin in 18 cats with squamous cell carcinoma resulted in 100% complete response rate and an overall 1-year control rate of 75%.58 Another study tested the efficacy of systemic pyropheophorbide-α-hexyl-ether for squamous cell carcinoma in cats; an alternative staging system was used in this report. Fifteen tumors were staged T1a (<1.5 cm diameter, noninvasive), 18 T1b (<1.5 cm, invasive), and 28 T2B (>1.5 cm, invasive). The tumor stage was of significant prognostic value for clinical outcome; complete response was achieved in 100% of T1a tumors, 56% of T1b tumors, and 18% of T2b tumors. The 1-year local control rate was 100% for T1a tumors and 53% for T1b tumors.348 Chemotherapy: Chemotherapy as a sole treatment has not led to long-term responses for cutaneous squamous cell carcinoma.624 Adjuvant chemotherapy combined with surgery for invasive lesions needs further investigation. Intralesional chemotherapy using intralesional sustained release cisplatin and 5-fluorouracil and carboplatin has been applied to dogs and cats with various stages of squamous cell carcinoma. Approximately 50% to 55% complete responses of various durations were achieved in dogs and 64% to 73% in cats.292,438,611 More recently, a combination of intralesional carboplatin and external-beam radiation therapy was applied in six cats with advanced stage (T2 to T4) nasal planum squamous cell carcinoma. All cats had a complete response, and four cats were disease free at a median follow-up of 268 days.118 Electrochemotherapy is an experimental treatment technique that uses locally applied electrical field pulses to induce an increased uptake of a systemically administered chemotherapeutic drug by cancer cells.77 It was used with the drug bleomycin to treat nine cats with T2 to T4 stage squamous cell carcinoma (mainly on the nasal planum); seven of nine cats had a complete response, which lasted more than 1 year in five of nine cats.573 Synthetic retinoids have been sparsely investigated for their effects on squamous cell carcinoma in dogs and cats in the past. Only preneoplastic lesions responded to etretinate (2 of 10 partial and 2 of 10 complete) in 10 dogs355 and a limited number of early superficial lesions responded to isotretinoin in a case of multiple squamous cell carcinoma in one dog that also received local hyperthermia.326 No significant response to 13-cis-retinoic acid was found in 10 cats.153 Cyclooxygenase-2 (COX-2) is overexpressed in many epithelial tumors,402,462 and inhibition of COX-2 using nonsteroidal antiinflammatory drugs (NSAIDs) has been proposed as adjuvant therapy for squamous cell carcinoma. Its efficacy has been clinically investigated in 17 dogs with high-stage oral squamous cell carcinoma (six dogs had lymph node metastasis, and one dog had distant metastasis). One complete response, two partial responses, and five dogs with stable disease resulted, with mean survivals of 223 (dogs showing a response) and 246 (stable disease) days.528 COX-2 expression has been detected in less than 1%230 and in 9% to 18% of feline oral squamous cell carcinoma25,123 but not in feline cutaneous squamous cell carcinoma.25 COX-1 overexpression was also found for oral squamous cell carcinoma in cats.230 One retrospective study investigated survival parameters of 54 cats with oral squamous cell carcinoma and found that the use of NSAIDs was associated with survival, although the study design permitted no conclusions about the effect of NSAIDs on prognosis.232 Effect of NSAIDs on cutaneous squamous cell carcinoma has not been investigated for dogs and cats.
Specific Disorders
Tumor Surgery of The Skin
General Considerations
Surgical Margins
EXCISION TYPE
PLANE OF DISSECTION
RESULT
Intralesional
Piecemeal debulking or curettage
Leaves macroscopic disease
Marginal
Shell out through or at the pseudocapsule or reactive zone
Usually leaves either microscopic or skip lesions
Wide
Entire lesion en bloc with a cuff of normal tissue
May leave skip lesions
Radical
Extracompartmental, en bloc with entire compartment (amputation)
No residual tumor
Biopsy
Surgical Principles
Factors Affecting Wound Healing
Radiation
Tumor-Related Factors
Tumor Staging and Patient Workup
Tumor Staging
STAGE
DESCRIPTION
T
Primary tumor
Tis
Preinvasive carcinoma (carcinoma in situ)
T0
No evidence of tumor
T1
Tumor <2 cm in diameter, superficial or exophytic
T2
Tumor 2–5 cm in diameter or with minimal invasion irrespective of size
T3
Tumor >5 cm in diameter or with invasion of the subcutis, irrespective of size
T4
Tumor invading other structures such as fascia, muscle, bone, or cartilage
N
Regional lymph nodes
N0
No evidence of regional lymph node involvement
N1
Movable affected ipsilateral node
N2
Movable affected contralateral or bilateral nodes
N3
Fixed nodes
M
Distant metastasis
M0
No evidence of distant metastasis
M1
Distant metastasis detected
Patient Evaluation
Cytology and Histology
Disease Extent
Lymphatic System
Lymphatics
Lymph Nodes
Lymphadenectomy in Surgical Oncology
Lymphangitis
Lymphedema
Diagnosis
Treatment
Neoplastic Skin Disorders
Tumor Classification
Papilloma
Squamous Cell Carcinoma
Etiology
Clinical Findings
Tumor Grade
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Specific Disorders
Only gold members can continue reading. Log In or Register to continue