Chapter 23
Short Tongue Syndrome and Hypovitaminosis A
Basic knowledge of amphibian nutrition lags behind that of reptiles and even fish. Awareness of worldwide amphibian population declines and extinctions and subsequent development of a variety of conservation-oriented captive propagation programs, as well as increased interest in amphibians for the pet trade, will increase the demand for improved nutritional management. Because the class Amphibia has such rich species diversity, development of a satisfactory “one size fits all” approach to dietary husbandry is unlikely; approaches that include careful observation of species’ natural history and experimental studies as have been described for reptiles will be necessary.1 Unfortunately, in many cases, advances in amphibian nutrition may come about only after the investigation of disease conditions that have features of nutritional disorders described in other taxonomic groups.
This chapter describes newly recognized conditions in captive amphibians that are suspected to be due to vitamin A deficiency. Vitamin A is a fat-soluble vitamin involved in a diverse variety of physiologic functions that include vision, epithelial cell differentiation, innate and adaptive immune response, reproduction, and bone development.2,3 Dietary sources of vitamin A include active forms from animal tissues (retinol) and provitamin forms (carotenoids) found in plants that are converted to active vitamin A in the intestinal mucosa. Vitamin A deficiencies are recognized in a variety of animal species, including domestic and nondomestic mammals, birds, reptiles, and fish, but had not been previously recognized in amphibians.2,4–8, The histologic finding considered to be characteristic of vitamin A deficiency is squamous metaplasia (SM) in which normal mucus-producing epithelium is replaced by a keratinizing stratified squamous epithelium.
SM of the mucous glands of the tongue was observed in association with a condition coined short tongue syndrome (STS) in the critically endangered Wyoming Toad (Anaxyrus [Bufo] baxteri).9 SM was clearly evident in affected toads because, unlike the normal keratinizing stratified squamous epithelium typical of the tongue in birds and mammals, the epithelium of the tongue in amphibians is nonkeratinizing, glandular, and mucus-producing, a feature that aids in capturing prey items10,11 (Figure 23-1). Subsequent investigation demonstrated that captive Wyoming Toads with SM had significantly lower liver retinol (vitamin A) levels when compared with wild Wyoming Toads, and the combined findings were considered to be highly suggestive of vitamin A deficiency. A review of the literature shows a single previous report of lingual SM in a long-term captive Pig Frog (Rana grylio) that was interpreted as a precancerous change or “epithelial malignancy” but, in retrospect, possibly represented a lesion associated with vitamin A deficiency.12
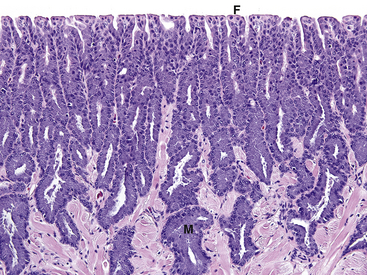
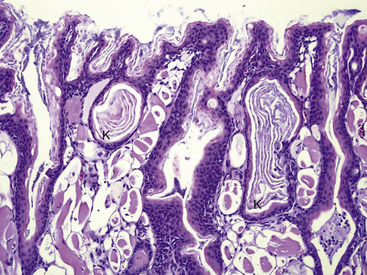
FIGURE 23-1 Histologic section of a normal tongue from a Wyoming Toad (Anaxyrus [Bufo] baxteri) (×20). Cells of the mucosal glands (M) are packed with a granular purple material (mucus). The dorsal surface has distinct filiform papillae (F).
SM of the tongue and symptoms of STS have also been observed in a variety of other captive anurans including Grey Foam-Nest Tree Frogs (Chiromantis xerampelina), Boreal Toads (Anaxyrus [Bufo] boreas boreas), Puerto Rican Crested Toads (Peltophryne lemur), Woodhouse’s Toads (Anaxyrus [Bufo] woodhousii), American Toads (Anaxyrus [Bufo] americanus), Panamanian Golden Frogs (Atelopus zeteki), and Kihansi Spray Toads (Nectophrynoides asperginis).9,13–15 Periocular and conjunctival swellings responsive to vitamin A supplementation have been observed in ranid and dendrobatid frogs, as well as a Tiger Salamander (Ambystoma tigrinum).16–18
Signalment and Clinical Signs
Because of the wide range of physiologic functions associated with vitamin A, it is worthwhile considering the possibility of hypovitaminosis A as a contributory factor in any sick captive amphibian maintained on a predominantly insectivorous diet. Potential clinical indicators at both an individual or population level could include bacterial or fungal infections occurring at levels greater than would be expected with good husbandry practices; poor reproductive success, including reduced egg viability and poor tadpole survival; failure to thrive; and hydrocoelom secondary to renal disease because SM of renal tubules has been observed in some animals with STS.
Diagnosis
Histopathology
Postmortem histopathologic evaluation for evidence of SM requires that the entire tongue be submitted in formalin and bisected longitudinally (median plane) before histologic processing. Early or mild SM of the lingual epithelium occurs rostrally in the dorsal mucus-producing glands and is observed in more caudal regions as lesions progress. Because of this specific lesion distribution, areas of SM can be easily overlooked if transverse sections from only the middle to caudal portions of the tongue are examined. Areas of SM in lingual glands can range from simple replacement of mucus-producing cells by larger pale-staining squamous epithelial cells to glands that are filled with laminated keratin (Figure 23-2 and Figure 23-3). In advanced lesions, SM can involve the entire dorsal mucous-producing surface of the tongue, as well as the ventral ciliated epithelium (see Figure 23-3). SM may not be observed in all animals with vitamin A deficiency; therefore the presence of this change is interpreted as a possible indicator of deficiency in a population, and the absence of SM should not be used to exclude the possibility of deficiency.19,20
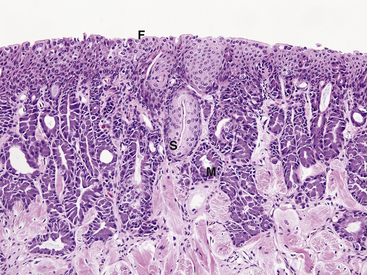
FIGURE 23-2 Histologic section of the tongue from a Wyoming Toad (Anaxyrus [Bufo] baxteri) with early squamous metaplasia (×20). Compare with Figure 23-1. Some mucosal glands are lined by large pale-staining squamous epithelial cells (S). Other mucosal glands still contain abundant cytoplasmic mucus (M). The dorsal filiform papillae are indistinct and fused (F).
Liver, Whole Body, and Serum Retinol Determination
In domestic animals, the liver is the principal site of vitamin A storage and is the preferred sample tissue for evaluation of suspected deficiencies.2,4 Published information on levels of liver retinol in amphibian species is limited, and interpretation of results for diagnostic purposes is based on comparison of values (if available) in normal free-ranging animals of the same or related species and, to a lesser degree, comparison of values reported for domestic animals.
For investigation of STS in Wyoming Toads, liver samples for determination of retinol levels were obtained from a variety of free-ranging toads including Wyoming Toads (available during a natural outbreak of chytridiomycosis), American Toads, Southern Toads (Anaxyrus [Bufo] terrestris), and a Boreal Toad (Table 23-1). The mean liver retinol values for 11 Wyoming Toads with STS and histologic evidence of lingual SM were 1.48 μg/g (range, not detectable to 7.3 μg/g) compared with a mean of 104.6 μg/g (range, 44 to 164 μg/g) for 10 free-ranging toads that had histologically normal tongues. In general, samples from all species of normal free-ranging toads examined had lower liver retinol values than ranges expected for domestic animals, but values still significantly exceeded those observed in toads with STS (see Table 23-1). A single free-ranging American Bullfrog (Lithobates [Rana] catesbeianus) had a liver retinol level of 12.5 μg/g, and this value is similar to those obtained for free-ranging bullfrogs in Canada (see Table 23-1).21 Based on limited information, a liver retinol value of less than 40 μg/g has been suggested as being suspicious for vitamin A deficiency, especially for bufonids; however, additional research on hepatic retinol levels in free-ranging amphibians is clearly indicated.17
TABLE 23-1
Liver Retinol in Wild Anuran Amphibians and Reference Ranges for Selected Domestic Animals
| Species/Number Examined (n) | Mean liver retinol μg/g (range) |
| Wyoming Toad (Anaxyrus baxteri) n = 10 | 104.6 wet (44.4-164)∗ |
| Southern Toad (Anaxyrus terrestris) n = 4 | 150.3 wet (96.4-242.6)∗,† |
| American Toad (Anaxyrus americanus) n = 2 | 493.5 wet (417.6-569.3)∗,‡ |
| Boreal Toad (Anaxyrus boreas boreas) n = 1 | 62.9 wet |
| American Bullfrog (Lithobates catesbeiana) n = 1 | 12.2 wet∗ |
| Domestic cattle | 400-700 dry§ |
| Domestic horses | 200-500 dry§ |
| Domestic chickens | 60-300 wet§,¶ |
∗Analyses performed by Dr. Ellen Dierenfeld, Wildlife Conservation Society.
†Samples provided courtesy of Dan Pearson.
‡Samples provided courtesy of Dr. April Paulman.
§From Puls R. Vitamin levels in animal health. Aldergrove, BC, Canada: Sherpa International, 1994;14-30.
¶Values cited as IU/g and converted to μg/g (1 IU = 0.3 μg).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree