Chapter 18
Analgesia
To put the issue into historical perspective, less than four decades ago, it was routine for human physicians to forego administering analgesic drugs to human infants, even after they had undergone invasive surgical procedures. Before the late 1970s, clinical and empiric research on human pain and pain management focused entirely on adults. Pain management for infants and young children was nonexistent, and surgery was typically performed with only paralytics on board. For example, one of the most common surgical procedures performed on premature babies was thoracotomy for ligation of a patent ductus arteriosus, which most surgeons and anesthesiologists believed was best accomplished with the use of oxygen and pancuronium as the sole anesthetic regimen.1 At the time, it was hypothesized that human infants had an immature central and peripheral nervous system, which was neither structurally nor functionally capable of receiving and processing noxious stimuli. Because infants are nonverbal, measurement of pain was considered difficult if not impossible. The thinking was that analgesic drugs not only would be ineffective but also would contribute to deleterious physiologic side effects, which would further jeopardize the recovery and, ultimately, the health of the child. During the 1980s and 1990s, clinical analgesia research focusing on human full-term and premature babies blossomed, and we now understand the following: nociceptive neural pathways are fully developed and in place in humans by 22 to 24 weeks’ gestation,2 and nonverbal human infants can respond to pain using vocalizations, facial expressions, body movements, changes in respiratory and heart rate, changes in skin color, and metabolic changes. With this in mind, is it any wonder that we are just beginning to understand nonhuman mammalian pain and analgesia? The acceptance of nonverbal human infants being able to perceive and express pain was a breakthrough and a prelude to a new focus on veterinary analgesia.
Many veterinary clinicians continue to argue that the administration of analgesics is risky to the patient and may mask behavioral signs of pain, which are considered evolutionarily adaptive for survival. This remains particularly true when nonmammalian patients are considered. This issue was expressed in a survey of members of the Association of Reptile and Amphibian Veterinarians.3 Most respondents (98%) believed that reptiles were capable of experiencing pain, but relatively few clinicians actually administered analgesics to reptiles under conditions in which they would administer analgesics to mammals. The implication from the survey data collected was that there were too many unknowns, recognition of pain was difficult, there was concern about detrimental side effects from analgesic administration, dosages and duration of effect were anecdotal, no pharmacokinetic data were available, and, at the time of the survey, published efficacy data were uncommon. Since that survey, we now recognize that, at some level, reptiles experience pain, and while there is interest in treating pain in reptile patients, we remain unsure if the chosen drugs, dosages, or duration of administration are effective or whether the chosen analgesic will have deleterious consequences. Added to this, the diversity of the class Reptilia makes it difficult to extrapolate analgesic efficacy from one species to the next. Regardless, veterinarians have an ethical obligation to treat painful conditions in all animals because effective pain management reduces stress-induced disruption to homeostatic mechanisms and also decreases morbidity and mortality associated with trauma or surgery. Therefore the primary objective of this chapter is to describe and highlight the most current information with respect to pain and analgesia in reptiles.
Reptile Nociception or Pain?
Do reptiles feel pain? Can we recognize pain in reptiles? Is the perception of pain by a reptile equivalent to that of a mammal? Does it make anatomic or physiologic sense that the ability to perceive and respond to an aversive stimulus is evolutionarily limited to mammals? We will never be able to objectively answer these questions because reptiles simply cannot tell us. However, like nonverbal human infants or nonhuman mammals, should the inability to communicate dictate whether pain is being perceived or whether an analgesic drug should be administered? I would argue, emphatically, no. By anthropocentric definition, the word pain implies higher level cortical processing of information; therefore nociception and antinociception are used when referring to pain and analgesia in most nonmammalian species. This stems from the controversy concerning whether nonmammalian species have the appropriate central and peripheral nervous system structures and pathways capable of “receiving and processing” noxious stimuli and responding appropriately. In other words, can nonmammals “experience” pain, or are they merely capable of demonstrating a “reflexive” response to a noxious stimulus, that is, nociception? Based on published neuroanatomic, neurophysiologic, and behavioral data, reasonable evidence suggests that, structurally and functionally, reptiles have the capacity to experience pain. Therefore, as clinicians, because of limited understanding of pain and analgesia in reptiles, we should err on the side of reptile patient well-being and make the assumption that conditions considered painful in humans and other mammals should be assumed to be painful across all other vertebrate species, including reptiles.
Neuroanatomic and Neurophysiologic Evidence
Nociceptive Pathways
It is adaptive for all animals to avoid aversive stimuli in the environment as an ultimate mechanism for survival; therefore all vertebrates have specialized sensory receptors, nociceptors that are capable of detecting noxious stimuli (e.g., thermal, mechanical, and chemical receptors), and afferent pathways relaying this information to the central nervous system. Efferent pathways exist to initiate a response, typically a movement away from the noxious stimulus. Therefore for any vertebrate organism to “perceive” a painful stimulus, the following must exist: a peripheral sensory receptor (e.g., nociceptor), a sensory pathway to the spinal cord, initial processing of the painful stimulus within the spinal cord dorsal horn, ascending pathways to the brain, processing of the painful stimulus by the brain, and descending pathways, which exert control over the withdrawal, escape, or defensive or immobile responses. Like mammals, reptiles have all of the anatomic structures considered critical for the recognition of pain: peripheral nociceptors, appropriate central nervous system structures and pathways, opioid receptors and endogenous opioids, reduction of nociceptive response with analgesics (although data are sparse), pain avoidance learning, and suspension of normal behavior with pain.4–6 Recent research in fish, amphibians, reptiles, and birds has demonstrated the transmission of peripheral sensory signals, via the spinal cord, to midbrain and forebrain regions that are homologous to mammalian cortical and limbic structures.7–10 Thus the physiologic and anatomic requirements for pain and analgesia appear to be remarkably similar among all vertebrate species.
Nociceptors
Although peripheral nociceptors have not been identified specifically in reptiles, nociceptors are highly conserved across phyla from invertebrates to fish, amphibians, birds, and mammals.11 Nociceptors have been identified in aquatic and terrestrial invertebrates, teleost fish, amphibians, and birds.9,10 The only reason nociceptors have not been specifically identified in reptiles is, simply, because nobody has looked.
Functionally, all nociceptors do not respond to the same stimuli. Some may be specifically mechanoreceptors, chemical receptors, or thermoreceptors, and some nociceptors may be multimodal and respond to a variety of different noxious stimuli. Unfortunately, Reptilia are among the least studied taxa with respect to the presence of nociceptors, nociceptive pathways, and central nervous system involvement. In Pit Vipers, touch and thermosensitive and thermomechanosensitive nociceptive neurons were identified in the trigeminal ganglia.12 Mechanonociceptors were identified in the cutaneous plantar nerve of the American Alligator (Alligator mississippiensis) and some of these mechanoreceptors also responded to a noxious thermal stimulus greater than 40°C.13
Ascending (Afferent), Cerebral Cortical, and Descending (Efferent) Pathways
We know from the mammalian literature that noxious sensory information is initially detected by cutaneous afferents, the cell bodies of which are in the dorsal root ganglia of the spinal cord, and can be separated into two main groups according to axon caliber and myelination: large diameter, myelinated A fibers and small diameter, unmyelinated C fibers.9 As with mammals, fish, amphibians, and reptiles have myelinated and unmyelinated afferent fibers running together in sensory nerves: large, myelinated A fibers (Aβ); small, myelinated A fibers (Aδ); and small, unmyelinated C fibers (C).10,14 In amphibians, small, slowly conducting fibers (Aβ and C) transmitted the majority of all impulses induced by noxious heat, pinching, pin pricks, and the application of dilute acetic acid to the skin.14 Limited published data exist for reptiles. In Pit Vipers, Aβ fibers were shown to respond to nonnoxious mechanical stimuli and to have larger somata, whereas Aδ fibers responding to noxious mechanical stimulation had smaller somata.12
Sensory information detected by nociceptors and carried by the cutaneous afferents is transmitted to the dorsal horn of the spinal cord and continues to the brain to activate systems responsible for producing the sensation of pain and the motivational-affective responses that accompany the experience of pain. In mammals, substance P, a peptide that is present in small primary afferents, nerves, and in the dorsal and ventral horns of the spinal cord, is expressed in direct association with painful stimuli. Substance P is highly conserved in mammalian and nonmammalian species, including invertebrates, fish, amphibians, reptiles, and birds. Specifically in reptiles, substance P is present in the nervous system of several turtle species.15,16
Transmission of sensory information moves from the spinal cord to the brain. In mammals, pain-related activity detected by the thalamus spreads to the insular cortex, (believed to process the feelings that distinguish, for example, pain from itching) and to the anterior cingulate cortex (believed to control the motivational element of pain). The transmission of nociceptive information from the peripheral to the central nervous system is likely the function of several different ascending pathways originating from neurons located within the spinal cord gray matter. The axons of these neurons travel within the spinal cord white matter, and they terminate in higher centers, including several parts of the thalamus and the brainstem reticular formation.17 In mammals, the ascending tracts in the anterolateral quadrant of the spinal cord that seem most likely to mediate pain are the spinothalamic, spinoreticular, and spinomesencephalic tracts.18 Neurons belonging to these pathways project chiefly contralaterally, and recordings in mammalian experiments have demonstrated the presence of nociceptive neurons among the cells of origin of all of these pathways.18 It was hypothesized that the sensory aspects of pain are mediated primarily by the spinothalamic tract and that the motivational-affective aspects result from activity in all three of these pathways. In reptiles, although the same basic ascending sensory pathways for the visual, auditory, and somatosensory systems are present, they involve fewer cell groups or subdivisions of cell groups in the thalamus and pallium compared with homologous pathways in birds and mammals.19–23
The telencephalon of reptiles, birds, and mammals consists of two major subdivisions: the pallium and the subpallium. The subpallium, also called the basal ganglia, is further divided into two main subdivisions: the striatum and pallidum. The striatum and pallidum are also thought to contribute to the septum and subpallial amygdala (central and medial nuclei). The subpallial components are relatively conserved in their organization among amniotes.24,25 The neocortex of mammals, as a derivative of the dorsal pallium, is homologous as a field only to the hyperpallium of birds and the dorsal cortex of lizards/turtles. The dorsal pallium is present in all amniotes and shares a number of similar cell types and connections across phyla.26 The organization of the reptile pallium is not yet as well-defined, but it consists of the dorsal cortex and the dorsal ventricular ridge (DVR), as well as olfactory, hippocampal, and pallial amygdala regions. The reptile anterior dorsal ventricular ridge (ADVR) is a large intraventricular protrusion in the reptilian forebrain, which receives information from many different sensory modalities and, in turn, projects massively into the striatum. The ADVR possesses functional similarities to the mammalian isocortex and may perform complex sensory integrations. The ADVR in lizards is composed of three longitudinal zones, which receive visual, somatosensory, and acoustic info. These projections are relayed by the thalamic nuclei.8 The posterior part of the dorsal ventricular ridge (PDVR) is considered an associative center that projects to the hypothalamus, thus being comparable to the amygdaloid formation. Evidence suggests that the PDVR and neighboring structures constitute the reptilian basolateral amygdala and indicate that an emotional brain was already present in the ancestral amniote.27
The descending motor pathways from the hypothalamus and brainstem to the spinal cord in the quadrupedal reptiles studied (turtles [Red-eared Slider, Trachemys scripta elegans, and Hermann’s Tortoise, Testudo hermanni] and lizards [Gold Tegu, Tupinambis nigropunctatus, and Savannah Monitor, Varanus exanthematicus]) appear to show remarkable similarities to pathways in mammals with regard to their cells of origin and their spinal cord trajectory.28 In the reptiles studied, the presence of interstitiospinal, vestibulospinal, and reticulospinal pathways could be demonstrated. The crossed reticulospinal tracts regulate the sensitivity of flexor responses to ensure that only noxious stimuli elicit the responses. A crossed rubrospinal tract has been shown in some turtles and lizards but could not be demonstrated in the Python.29 A small rubrospinal tract was demonstrated in a colubrid snake species (Watersnake, Nerodia sp).30 The nonexistent or reduced rubrospinal tract in snakes is believed to be associated with limblessness. In the Large Psammodromus, efferent fibers from the dorsal cortex reach the rostral midbrain tegmentum, and it was suggested that this was the reptilian representation of the sensorimotor cortex.31 A similar corticoefferent pathway was described in turtles after lesions in the general cortex.31 In Tokay Geckos (Gekko gecko), anterograde-labeled fibers were observed in the same tegmental area after a tracer injection into different locations of the dorsal cortex.31 Although axon terminals were not described in the Tokay Gecko midbrain tegmentum, thus far reptile data strongly suggest the presence of a corticoreticulospinal motor pathway. This pathway originates from the rostral dorsal cortex, and it may represent the anatomic substrate for a motor pallium in reptiles, which is comparable to the avian “motor pallium,” both topologically and with respect to the organization of efferent pathways.31 In addition, in Red-eared Sliders, the determination of a descending pathway linking cortical regions with the red nucleus via the hypothalamus suggested indirect cortical control of the reptilian rubrospinal system.32 Altogether, these findings suggest that the presence of functionally segregated thalamocortical projections is a conserved feature of brain organization among amniotes.
Opioid Receptors and Endogenous Opioids
The opioid receptor gene family is highly conserved across multiple vertebrate orders (e.g., bovids, chickens, bullfrogs, and teleost and elasmobranch fishes),11 but there is limited information on opioid receptors in reptiles. The three main opioid receptors, μ, δ, and κ, were cloned and sequenced in three nonmammalian vertebrates: the Zebrafish (Danio rerio), the Northern Leopard Frog (Rana pipiens), and the Rough-skinned Newt (Taricha granulosa).33–35 In aquatic turtles, μ- and δ-opioid receptors are located throughout the brain, and δ-opioid receptors are more abundant than μ-opioid receptors.36 However, results of the study did not determine the location and distribution of μ- and δ-opioid receptors in the spinal cord (where nociceptive inputs enter the central nervous system) of turtles, nor were κ-opioid receptors examined anywhere in the central nervous system. With respect to endogenous opioid-related neurotransmitters, proenkephalin-derived peptides are present in turtles with a distribution similar to that in mammals and birds.37 In addition, the reptilian brain (aquatic slider turtles, American Alligators, and anole lizards) was found to contain large quantities of endogenous enkephalins, also known as endorphins (Met-enkephalin, Leu-enkephalin, and Met-enk-Arg-Phe [MERF]).38
Measurement and Quantification of Pain and Analgesia in Reptiles
Measuring pain in any species, particularly reptiles, is the most difficult hurdle in the study of pain and analgesic efficacy. For example, as veterinarians, do we assume that this Bearded Dragon or that sea turtle is experiencing some level of pain and should we attempt to provide an analgesic to make them more comfortable (Figures 18-1 and 18-2)? In mammals, it is well-established that perioperative pain management facilitates recovery and healing, reduces morbidity and mortality, and contributes to more rapid return to normal behavior.39–41
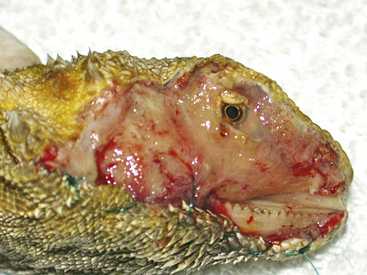
FIGURE 18-1 Bearded Dragon (Pogona vitticeps) with complete skin sloughing of the right side of the face due to Chrysosporium anamorph of Nannizziopsis vriesii (CANV) infection.

FIGURE 18-2 Green Sea turtle (Chelonia mydas) shortly after being struck by a boat propeller; the turtle was being treated at the Georgia Sea Turtle Center, St. Catherines Island, Georgia. (Courtesy Dr. Terry M. Norton, Georgia Sea Turtle Center, Jekyll Island, Ga.)
Why not consider this as salient in nonmammalian species? If measuring pain in mammals is challenging, how do we begin to decipher pain-related behavior in reptiles? An objective understanding of normal behavior of a particular species and the ability to differentiate the presence of abnormal behavior indicative of discomfort are critical to the study of pain and analgesia. Therefore one must first have an understanding of normal species-specific behavior within the environmental context in which that behavior is being displayed or observed, to be able to discriminate behavior associated with pain. For example, observing the postovariectomy behavior of an adult female Green Iguana in its own home cage may be different than behavior of the same animal under the same postovariectomy conditions observed in a hospital setting. This is certainly true in some mammalian species.42 Methods for assessing and measuring pain in reptiles have been described previously.41 Ideally, a combination of appropriate behavioral and physiologic variables might best be used to measure pain and analgesia in reptiles. Along those same lines, the development of a species- and context-specific ethogram for each species being evaluated would provide the best method for distinguishing normal versus abnormal (e.g., painful) behaviors. Most commonly, animal pain, or lack thereof, is assessed before and after some invasive procedure, such as a surgical procedure. This method requires the development of a behavioral ethogram, which, in turn, requires the observer to become well-versed in subtle behavioral differences through many hours of observation and analysis (videotaped or live observation). Behaviors must be operationally defined, which will provide objectivity and reproducibility (Box 18-1). For example, in a recent study, our laboratory developed a behavioral ethogram to evaluate preoperative and postoperative behavioral responses to food intake, willingness to swim, and breathing in Red-eared Sliders after unilateral orchidectomy.43 We were able to compare postoperative behavior in turtles with and without analgesic administration of morphine, butorphanol, and saline. Our hypothesis was that preoperative behaviors would return to normal more rapidly in those turtles receiving a μ-opioid receptor agonist analgesic. We demonstrated that those turtles receiving morphine and undergoing unilateral gonadectomy returned to normal preoperative behavior more quickly than those receiving saline or butorphanol. An alternative to studying postsurgical pain is to measure pain under strictly controlled laboratory conditions with the use of established behavioral models during which noxious stimuli (e.g., mechanical, thermal, or chemical) are applied to an anatomic location on the reptile subject.44–48











