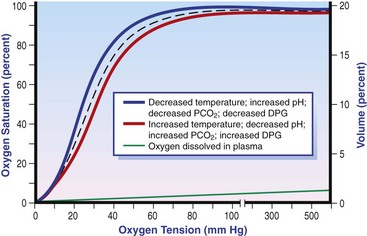
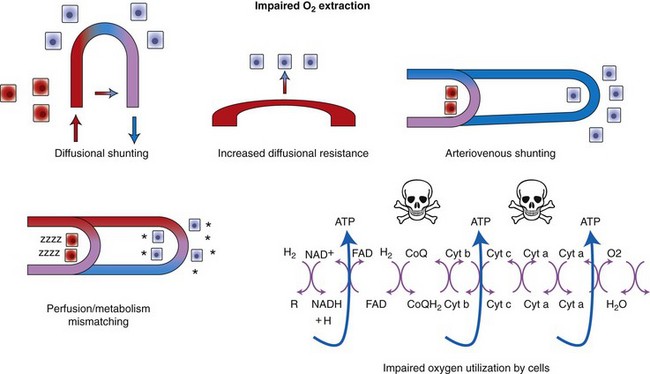
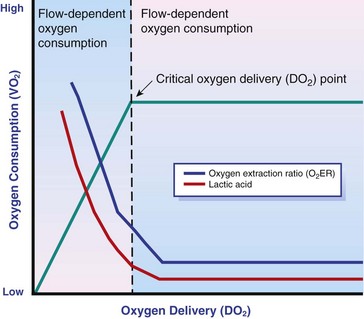
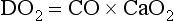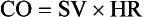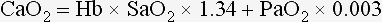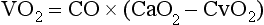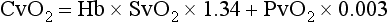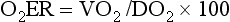Chapter 6 Shock can be classified according to its main contribution to impaired tissue oxygenation (Box 6-1 and Table 6-1). Most forms of shock impair oxygen delivery. Cardiogenic shock is used to describe conditions that impair forward flow of blood from the heart. Although sometimes described as “obstructive shock,” conditions that restrict right ventricular filling may also be grouped under cardiogenic shock. Hypovolemic shock occurs when circulating volume is inadequate. Distributive shock is caused by inappropriate vasodilation, resulting in inadequate effective circulating volume. Septic shock is one form of distributive shock, but its pathophysiology is complex, and it can result in impairment of cardiac function, fluid loss, and impaired oxygen utilization. Hypoxic shock results from inadequate arterial oxygen content or impaired mitochondrial function (impaired oxygen uptake). A list of abbreviations and equations used in this chapter is provided in Tables 6-2 and 6-3. Table • 6-2 Abbreviations Used in This Chapter Table • 6-3 The solubility of oxygen in plasma is low; therefore, the major determinant of arterial oxygen content is hemoglobin. The ability to carry oxygen is influenced by the amount of hemoglobin. The saturation of hemoglobin is dependent on the function of the hemoglobin molecule (i.e., its ability to take up oxygen in the lung and release it into the tissues) and of gas exchange in the lung (Figure 6-1). Cardiac output is a reflection of the ability of the heart to pump blood through the lungs for oxygenation, and then through the remainder of the body for oxygen delivery, and is influenced by heart rate (HR) and stroke volume (SV). Cardiac output is the volume of blood ejected by the heart into the systemic circulation each minute. Cardiac output is calculated from the product of stroke volume and heart rate. In human patients, cardiac output is measured in L/min. However in veterinary patients, because of the highly variable sizes and shapes of patients, cardiac output is often expressed in terms of mL/kg/min, or as the correlated cardiac index.36 Cardiac index is obtained by dividing the cardiac output by the body surface area and is therefore expressed as mL/m2/min. Because the pulmonary and systemic circulations occur in series, in normal physiology, the cardiac output of the left and right heart is equivalent. Acute increases in cardiac output occur through increases in heart rate. Tachycardia is effective for short-term increases in cardiac output, but it occurs at a cost. Increases in heart rate require shortening of the cardiac cycle. Systole requires a fixed amount of time; therefore diastole is disproportionately reduced during tachycardia. The consequences of shortened diastole include reduced filling time, which can limit stroke volume and reduce cardiac perfusion (the coronaries are perfused during diastole). Most chronic responses to increased cardiac output rely on increases in stroke volume. Factors that determine stroke volume are preload, afterload, and contractility. Oxygen (O2) content depends mainly on hemoglobin concentration (in g/dL) and oxygen saturation of hemoglobin in arterial blood (SaO2; measured as %). SaO2 is the percentage of available hemoglobin that is bound to oxygen. Oxygen binds hemoglobin in a cooperative relationship (i.e., hemoglobin affinity for oxygen increases as the oxygen saturation of hemoglobin increases). For this reason, the oxygen-hemoglobin dissociation curve, which relates hemoglobin saturation of O2 to the partial pressure of oxygen (PO2), is a sigmoidal curve (see Figure 6-1). Hemoglobin affinity for oxygen is influenced by factors such as pH, temperature, 2,3-diphosphoglycerate (2,3-DPG), and CO2 that can cause a shift in the dissociation curve, making O2 more or less readily released into the tissues. Stroke volume is influenced by preload, afterload, and contractility. The classic example of a reduction in preload is hemorrhagic shock; loss of whole blood results in absolute hypovolemia and an inability to adequately fill the ventricle (Box 6-2). Hypovolemia can also result from loss of electrolyte-rich fluid (e.g., with profuse vomiting and diarrhea) or loss of pure water (e.g., in heatstroke and adipsia). In addition to external loss, fluids can be sequestered internally in “third spaces,” in which fluid is not available to replenish the intravascular volume. Examples of third-spacing include ascites, pleural effusion, and fluid sequestration in the gastrointestinal tract. End-diastolic volume can also be limited by impaired right ventricular filling. A “tension pneumothorax” increases intrathoracic pressure and results in collapse of the great vessels and right ventricle. Pericardial effusion can lead to cardiac tamponade and collapse of the chambers of the right heart. Restrictive cardiac disease will also impair diastolic function. Afterload is typically maintained in compensated hypovolemic and cardiogenic shock. The normal response to preserve vital organ perfusion is vasoconstriction of peripheral vascular beds. Clinically, this is apparent as cool extremities, pale mucous membranes, and prolonged capillary refill time. Abnormal (decreased) afterload is the primary mechanism that leads to distributive shock. Circulating mediators, including nitric oxide, prostanoids, and complement, lead to inappropriate vasodilation, reduction of effective circulating volume, hyperemic mucous membranes, and rapid capillary refill time. Impaired contractility can result from primary cardiac disease, drugs, myocardial ischemia (as can occur with persistent tachycardia), or circulating inflammatory mediators. Cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1-beta exert short-term direct depressant activity over the myocardium and may contribute to cellular signaling events (e.g., apoptosis, nitric oxide pathway activation, calcium homeostasis imbalance, reduced response to catecholamines) that lead to the prolonged cardiac dysfunction seen in sepsis.75 Adequate arterial oxygen content is essential to maintain oxygen delivery. Three main categories of abnormalities can result in reduction of arterial oxygen content: anemia, altered hemoglobin function, and hypoxemia (reduced SpO2 [oxygen saturation of hemoglobin in peripheral blood as measured by pulse oximetry] or reduced SaO2). Blood loss, red cell destruction, or reduced red cell synthesis can affect the amount of hemoglobin in the blood, leading to anemia and decreased arterial oxygen content. Even in the face of adequate amounts of hemoglobin, arterial oxygen content can be reduced as a result of impaired hemoglobin function. Toxins, such as carbon monoxide, can impair the ability of hemoglobin to carry O2, which leads to tissue hypoxia. Shock is typically a state of acidemia that results in a decreased hemoglobin affinity for oxygen, which will decrease the ability of hemoglobin to take up O2 but enhances the release of O2 in the tissues. Hyperthermia, hypercarbia, and increased 2,3-DPG also result in decreased O2 affinity (see Figure 6-1). In profound cases of shock, hypothermia can complicate delivery of oxygen to the tissues because of the increased O2 affinity of hemoglobin. If hemoglobin is adequate and is able to carry and release O2, but arterial oxygen content is low, then impairments in pulmonary function and gas exchange are likely culprits. Hemoglobin saturation of venous blood, or SvO2, should be measured on a sample collected from the pulmonary artery (mixed venous oxygen tension [PvO2]). Mixed venous samples are representative of global VO2. Peripheral venous samples reflect the VO2 of the organs being drained and can be highly variable. Although the correlation between mixed venous and central venous (cranial vena cava) hemoglobin saturation is variable, for clinical purposes, mixed venous oxygen saturation is often substituted by central venous oxygen saturation (ScvO2).81 ScvO2 has been recognized as a valuable endpoint of resuscitation.82 If oxygen delivery is adequate, sufficient O2 should remain in the venous blood to provide at least 70% saturation of the hemoglobin. Although low ScvO2 can be attributed to low DO2 and low tissue oxygenation, five commonly described defects occur in VO2 in which the ScvO2 can be adequate, but tissue oxygenation can be impaired (Box 6-3). Oxygen extraction ratio (O2ER) is the ratio between oxygen uptake and oxygen delivery expressed as a percentage; it represents an index of the efficacy of tissue extraction of oxygen from the capillary bed. Oxygen extraction ratios of different organs are extremely variable in physiologic and pathologic conditions. Global O2ER under resting physiologic conditions is approximately 20% (20.5 ± 5.7 in dogs)36 but can increase to 60% to 70% in cases of increased metabolic demand and/or a reduction in oxygen delivery.102 Variation in O2ER represents an adaptive system that allows metabolism to maintain a constant VO2 over a wide range of DO2 variability without compromising aerobic metabolism. Oxygen delivery and uptake are interrelated through a bi-linear relationship (Figure 6-2).47 Over a wide range of values, VO2 remains constant and independent of DO2. This relationship corresponds to the plateau of the graph and is defined as O2 supply–independent VO2. The plateau is not completely flat because the metabolism of some organs, such as the kidney, is partially flow dependent. As oxygen supply decreases, VO2 remains stable initially as a result of increased oxygen extraction. However, below a critical oxygen delivery (cDO2), the increase in oxygen extraction is no longer able to match aerobic metabolism requirements, and oxygen consumption becomes dependent on its supply (supply-dependent VO2). cDO2 is known as the anaerobic threshold, because at cDO2, energy production through oxidative pathways is limited, prompting a shift toward a much less efficient anaerobic metabolism. During hypoxia, lack of oxygen leads to an upstream blockage in the mitochondrial electron transport chain, preventing the entry of pyruvate into the Krebs cycle. In this setting, cell energy production relies exclusively on anaerobic glycolysis. To avoid the accumulation of byproducts that would slow the glycolytic reaction, pyruvate is converted to lactate by the enzyme lactate dehydrogenase (LDH). This reaction regenerates NAD+, a necessary coenzyme for continued anaerobic glycolysis, thereby preserving adenosine triphosphate (ATP) production. When oxygen is present, lactate can be converted back to pyruvate or glucose and used as a source of energy in several organs, including heart, brain, liver, and skeletal muscle. However, if the hypoxia is global, lactate will diffuse into the bloodstream. Major sources of lactate are muscle and the gastrointestinal tract. Hyperlactatemia occurs when lactate generation exceeds its clearance by metabolism, primarily in the liver and kidneys.4 Hyperlactatemia induced by tissue hypoxia is commonly associated with metabolic acidosis. However, lactate production from glucose does not generate hydrogen ions (H+). During anaerobic metabolism, concurrent accumulation of H+ is derived from changes in other metabolic pathways. The degradation of ATP to adenosine diphosphate (ADP) produces H+ that under aerobic conditions are consumed by mitochondrial ATP production. In anaerobic conditions, these H+ accumulate in parallel with lactate, creating an acidemic state commonly referred as metabolic lactic acidosis.54 Inflammation in shock can be triggered by the inciting cause of shock, such as bacterial components in septic shock. Other forms of shock also trigger inflammatory responses through hypoxia. Cell death from energy failure and loss of electrical and chemical gradients will result in the release of endogenous danger signals (also referred to as danger-associated molecular patterns, or DAMPs).52 These danger signals are typically intracellular components that when released into the extracellular space bind to receptors on cells of the innate immune system and trigger an inflammatory response. In addition, exposure of cells to hypoxia can initiate activation of transcription factors (i.e., hypoxia-inducible factors; Box 6-4) that are responsible for the adaptive response to hypoxia, as well as activation of inflammatory mediators. Another cause of inflammation in shock is the reintroduction of oxygen to previously hypoxic or ischemic tissues. This reperfusion injury is associated with the generation of damaging oxygen and nitrogen free radicals. When tissues are ischemic, blood flow is compromised, resulting in decreased delivery of oxygen and nutrients. Ischemic tissues become hypoxic, consume the available nutrients, and accumulate waste products like lactate. The energy supply in the form of ATP is depleted, and the byproducts, inosine and hypoxanthine, accumulate. Intracellular calcium increases, which results in the activation of calpain, a proteolytic enzyme. The enzyme that normally metabolizes hypoxanthine, xanthine dehydrogenase, is converted by calpain to xanthine oxidase. During the ischemic phase, hypoxanthine accumulates because xanthine oxidase requires molecular oxygen. With the reintroduction of oxygen, xanthine oxidase metabolizes hypoxanthine and generates superoxide, a potent free radical. In addition, during ischemia, the cell responds to hypoxia and switches from aerobic to anaerobic metabolism through the activation of hypoxia-inducible factors (see Box 6-4). In addition to the compensatory mechanisms that are activated, several inflammatory mediators are induced by hypoxia. Nitric oxide synthase, which also requires oxygen for the production of nitric oxide nitric oxide, accumulates, and when oxygen is reintroduced, nitric oxide production can occur rapidly. Nitric oxide, a reactive nitrogen species, can rapidly interact with superoxide and form peroxynitrite. Upon reperfusion, the production of reactive oxygen and nitrogen species causes tissue damage and the release of increased numbers of cytokines and other inflammatory mediators. Neutrophils and endothelial cells are activated, further amplifying the damage and obstructing capillaries and causing a “no reflow” phenomenon that prevents normal perfusion.58,60 Hemostatic derangements play a major role in the pathophysiology of shock, independent of the inciting cause of shock. Altered coagulation appears early in the course of shock, especially when shock is caused by substantial tissue injury (trauma) or a systemic inflammatory process (sepsis). Several mechanisms contribute to hemostatic derangements37: • Tissue trauma favors coagulation and concurrent fibrinolysis. Endothelial damage and exposure of subendothelial collagen and tissue factor activate the coagulation cascade. Endothelial damage also induces a hyperfibrinolytic state due to increased synthesis and release of tissue plasminogen activator (tPA). • Shock, through hypoperfusion, enhances the effects of tissue trauma and promotes an anticoagulant and hyperfibrinolytic state. These hemostatic derangements result from ischemia-induced endothelial release of tPA, inhibition of plasminogen activator inhibitor (PAI)-1, and increased activation of protein C. Tissue trauma AND shock are the main contributors to coagulopathy in the early phases of shock. • Inflammation may be caused by the inciting cause of shock (sepsis) or may appear later in the course of shock as a consequence of tissue hypoxia and ischemia-reperfusion injury. Inflammation and coagulation are closely interrelated by a bidirectional cross-talk.48 Proinflammatory cytokines induce the expression of tissue factor by mononuclear cells and may directly activate platelets, causing a systemic procoagulant effect. Inflammation contributes to activate coagulation, also through the downregulation of all major anticoagulant pathways (i.e., antithrombin, activated protein C, and tissue factor pathway inhibitor). Coagulation factors (e.g., thrombin) and anticoagulant factors (e.g., activated protein C) may, in turn, affect inflammation by interacting with endothelial and mononuclear cell receptors and modulating cytokine expression and cellular interactions. Systemic inflammation induces a prothrombotic state and eventually will lead to disseminated intravascular coagulation (DIC). • With the evolution of shock, other factors aggravate the coagulopathy. Hypothermia is common in advanced stages of shock and inhibits platelet aggregation and coagulation factor activity. Acidosis, associated with tissue hypoperfusion, impairs coagulation factor activity. Dilution of coagulation factors may occur as a result of fluid shifts and fluid therapy during resuscitation. Moreover, some resuscitative fluids (synthetic colloids) can directly interfere with hemostasis. Hemodynamic and metabolic derangements associated with shock are sensed by several different mechanisms that activate a compensatory response. The goal of this response—to support vital organs with adequate oxygen delivery—is achieved through several different mechanisms44: • Maintaining mean circulatory pressure (circulating volume and pressure) • Maximizing cardiac performance • Constriction of most of the arterioles, leading to an increase in systemic vascular resistance and redirection of blood flow toward vital organs • Constriction of large capacitance venules and veins, resulting in an increase in venous return and a reduction in the effective vascular volume (in normal conditions, reservoir veins contain 60% of the circulating volume) • Marked increase in heart rate, with a consequent increase in cardiac output
Shock
2,3-DPG
2,3-Diphosphoglycerate
ABP, mm Hg
Arterial blood pressure
ACTH
Adrenocorticotropic hormone
ADH
Antidiuretic hormone
ADP
Adenosine diphosphate
aPTT
Activated partial thromboplastin time
ARDS
Acute respiratory distress syndrome
ARNT
Aryl hydrocarbon nuclear translocator
ATP
Adenosine triphosphate
AVP
Vasopressin (also called antidiuretic hormone, or ADH)
CaO2, mL/dL
Arterial oxygen content
cDO2, mL/min
Critical oxygen delivery
CO, L/min
Cardiac output
CO2
Carbon dioxide
CRH
Corticotropin-releasing hormone
CvO2, mL/dL
Venous oxygen content
CVP, mm Hg
Central venous pressure
DAP, mm Hg
Diastolic arterial pressure
DIC
Disseminated intravascular coagulation
DO2, mL/min
Oxygen delivery
ECG
Electrocardiogram
EDP
End-diastolic pressure
EDV
End-diastolic volume
FAST
Focused assessment with sonography for trauma
FiO2
Fractional inspired oxygen
H+
Hydrogen ion
H2O
Water
Hb, g/dL
Hemoglobin
HCO3−
Arterial bicarbonate
Hct, %
Hematocrit
HIF
Hypoxia-inducible factor
HMGB
High mobility group box protein
HR, bpm
Heart rate
HRE
Hypoxia response element
ICAM
Intercellular adhesion molecule
IL
Interleukin
LDH
Lactate dehydrogenase
LiDCO
Lithium dilution cardiac output
MAP, mm Hg
Mean arterial pressure
MODS
Multiple organ dysfunction syndrome
NaCl
Sodium chloride
NAD+
Nicotinamide adenine dinucleotide
NADH
Coenzyme formed by reaction of NAD+ with oxidizing agent
O2
Oxygen
O2ER, %
Oxygen extraction ratio
OPS
Orthogonal polarization spectral imaging
PAI-1
Plasminogen activator inhibitor
PaO2, mm Hg
Arterial oxygen partial pressure
PCO2
Partial pressure of carbon dioxide
PCV
Packed cell volume
PiCCO
Transpulmonary thermodilution cardiac ouput
PIRO
Predisposition, infection, response, organ failure
PO2
Partial pressure of oxygen
PT
Prothrombin time
PvO2
Mixed venous oxygen tension
rhaPC
Recombinant human activated protein C
RR
Respiratory rate
SaO2, %
Arterial hemoglobin oxygen saturation
SAP, mm Hg
Systolic arterial pressure
ScvO2, %
Central venous oxygen saturation
SIRS
Systemic inflammatory response syndrome
SpO2, %
Oxygen saturation of hemoglobin in peripheral blood as measured by pulse oximetry
StO2
Muscle tissue oxygen saturation
SV, mL
Stroke volume
SvO2,%
Hemoglobin saturation of mixed venous blood
SVR
Systemic vascular resistance
TNF
Tumor necrosis factor
tPA
Tissue plasminogen activator
VO2, mL/min
Oxygen uptake
Oxygen delivery
DO2 (mL/min) = CO (L/min) × CaO2
Cardiac output
CO = SV × HR
Arterial oxygen content
CaO2 = Hb × SaO2 × 1.34 + PaO2 × 0.003
Venous oxygen content
CvO2 = Hb × SvO2 × 1.34 + PvO2 × 0.003
O2ER
O2ER = VO2/DO2 × 100
MAP
DAP + 1/3 × (SAP − DAP)
Pathophysiology of Impaired Oxygen Delivery and Oxygen Uptake
Determinants of Oxygen Delivery
Arterial Oxygen Content
Defects in Oxygen Delivery
Oxygen Uptake
Oxygen Extraction Ratio
DO2/VO2 Curve
Cellular Response to Hypoxia and Lactate
Pathophysiology of Shock
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree