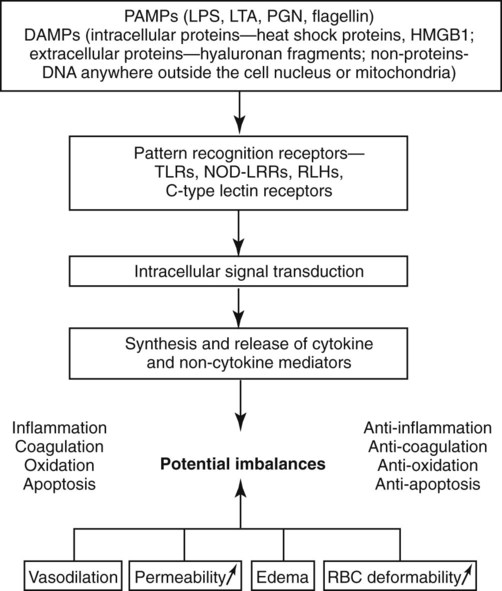
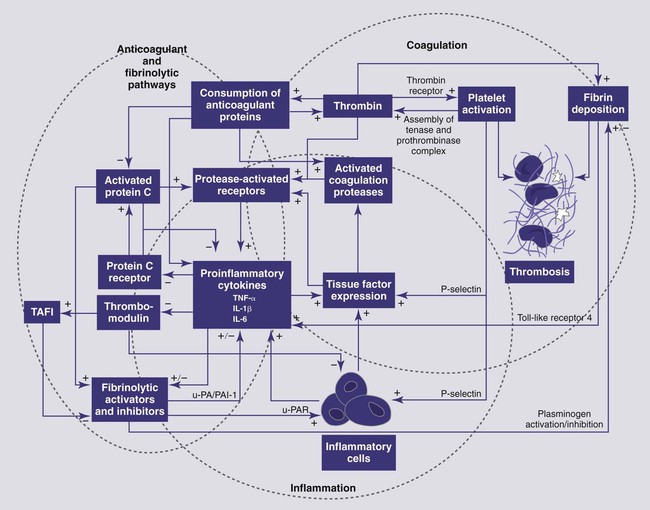
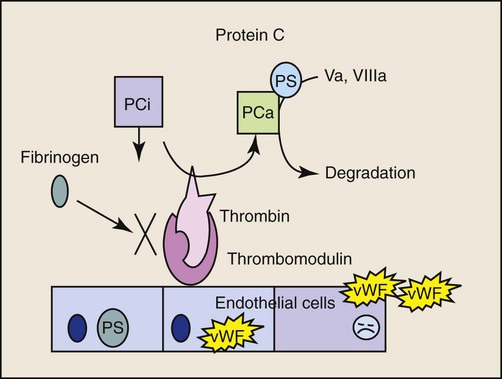
Bacteremia is defined as live microorganisms in circulation. Endotoxemia refers to circulation of the lipopolysaccharide (LPS) component of the gram-negative bacterial cell wall and may or may not be associated with the presence of live bacteria. Sepsis represents the clinical signs associated with a systemic inflammatory response to an infectious agent.13 Bacteremia in dogs and cats is expected to occur in a variety of infections (e.g., peritonitis, mastitis, prostatitis, pneumonia, pulmonary abscess, pyothorax, pyometra, pyelonephritis, intra-abdominal abscess, biliary tract infections), as well as during dentistry, but is not always associated with a systemic inflammatory response.73 Subclinical bacteremia probably also occurs during rectal palpation, intestinal endoscopy and surgery, and when indwelling venous or arterial catheters have been placed. Normally, circulating bacteria are cleared by the mononuclear-phagocyte system; therefore, blood cultures are not always reliable in documenting bacteremia. Bacterial molecules, along with molecules from other infectious agents (e.g., fungi, virus), are referred to as “pathogen-associated molecular patterns” or PAMPs.103 Macrophages and other cells of the innate immune system recognize these PAMPs through Toll-like receptors (TLRs) and initiate a complex local inflammatory response and upregulate systemic host defenses, thus preparing the animal to combat potential spread of infection. Typical clinical signs caused by bacteremia include altered body temperature, tachycardia, tachypnea, and neutrophilia, or neutropenia; these signs represent the systemic inflammatory response syndrome (SIRS) which when initiated by an infectious agent is referred to as sepsis. Not all patients with bacteremia will demonstrate clinical signs of sepsis.73 SIRS is characterized by a complex cytokine-mediated response and can also be initiated by endogenous danger signals (danger-associated molecular pathogens or DAMPs) from damaged cells as a result of trauma, pancreatitis, hypoxia, and other insults.103 The host benefits if the inflammatory response remains localized and balanced by anti-inflammatory pathways; however, systemic inflammation or failure to resolve the inflammatory response can lead to high mortality. The terminology SIRS and sepsis was clarified in the 1990s in human medicine13; relevant terms and the various categories of severity are defined in Table 36-1. SIRS criteria, however, are very insensitive and nonspecific features of sepsis that were designed to identify patients eligible for clinical trials of antisepsis therapies (Tables 36-2 and 36-3). A more recent human consensus conference98 has identified a more elaborate classification system (PIRO) that focuses on host factors (predisposition), infectious agent factors (infection), the host-organism interaction (response), and the consequences of systemic inflammation (organ failure). See Web Table 36-1 for details of this system. The inappropriate, dysregulated generalized systemic response leads to a progression of severe sepsis, septic shock, and ultimately multiple organ dysfunction syndrome (MODS).90a TABLE 36-1 Definitions of Systemic Inflammatory Response Syndrome and Sepsisa TABLE 36-2 Systemic Inflammatory Response Syndrome Criteria for Dogs, Cats, and Humans TABLE 36-3 Various Proposed Canine Systemic Inflammatory Response Syndrome Criteria WEB TABLE 36-1 PIRO System for Staging Sepsis HLA-DR, Human leukocyte antigen-DR; IL, interleukin; LPS, lipopolysaccharide; PAF, platelet activating factor; PCT, procalcitonin; PIRO, predisposition, infection, response, organ dysfunction; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment score; TNF, tumor necrosis factor. From Ref. 98. Much of our knowledge of sepsis has been derived from experimental animal models and human (and a few veterinary) clinical studies. Animal models of sepsis can be divided by the type of insult, the route of administration of the infectious agent or substance, and the endpoints of the study.26 One of the most commonly used agents to study the physiologic response to systemic bacteria has relied on the use of endotoxin. Endotoxin (LPS) consists of a lipid A moiety (the toxic component) and polysaccharides (O antigens). LPS is released from the outer membrane of gram-negative bacteria when they lyse and is normally confined within the gut and portal circulation. The source of endotoxin is usually a member of the Enterobacteriaceae family (Escherichia spp., Klebsiella spp., Enterobacter spp., Proteus spp.), as well as Pseudomonas spp. Studies of endotoxemia are highly reproducible, but administration of endotoxin to animal models does not account for all of the factors involved in clinical conditions in which endotoxin is a component of the disease. The most primitive form of the immune system, the innate immune system, has evolved to recognize and respond to LPS in tissues and fluids early in gram-negative infections.142 A major function of the innate immune system is to recognize threats to host survival. These threats can be exogenous or pathogen associated (PAMPs), or endogenous danger signals released from damaged or dying cells (DAMPs).103 The pathway for recognition of endotoxin has been well defined and is the prototypical example of activation of the innate immune response. The innate immune system is also capable of recognizing gram-positive bacterial products, flagellin, bacterial or viral DNA, and fungal products through TLR.4,100 Web Table 36-2 lists pathologic microorganism products that are recognized by the immune system. WEB TABLE 36-2 Microorganism Features That Are Recognized by the Toll-like Receptors CpG, Regions of DNA where a cytosine (C) nucleotide occurs next to a (G) guanine nucleotide, where p represents the phosphodiester backbone, found abundantly in bacterial DNA; GPI, glycosylphosphatidylinositol; HSP60, heat shock protein 60; LPS, lipopolysaccharide; mRNA, messenger RNA; TLR, toll-like receptor. The prevalence of naturally occurring sepsis in dogs and cats is unknown, even though the dog has been used for many years as a biomedical model of sepsis, endotoxemia, and SIRS for people.137 Risk factors for endotoxemia, with or without concurrent bacteremia, in dogs include canine parvovirus (CPV) enteritis, gastric dilation and volvulus, pyometra, mastitis, other gram-negative infections, and heat stroke. Endotoxemia can develop when hepatic clearance of LPS is reduced, as in hepatic insufficiency and portosystemic shunts. Obstructive biliary disease may be a risk factor because bile salts bind endotoxin in the gut. Endotoxin may also play an important role in the pathogenesis of severe acute pancreatitis in dogs.165 In the dog, sepsis is most commonly associated with disruption of the normal mucosal barrier and movement of bacteria into the circulation or tissues. The most commonly reported causes of sepsis in dogs include peritonitis (from gastrointestinal [GI] or urogenital origins) and pneumonia.16,45,45Dogs receiving chemotherapy are susceptible to develop sepsis.187a Smaller dogs with lymphoma, after receiving doxorubicin, or vincristine are at highest risk. Sepsis is recognized less commonly in cats. Clinically affected cats, particularly those with severe sepsis, do not commonly display the classic hyperdynamic signs of sepsis (tachycardia, red mucous membranes, fever, bounding/hyperkinetic pulses), making diagnosis a challenge.25,164 In an experimental study of low-dose endotoxin infusion, the cats developed hypotension but not tachycardia.44 Risk factors for severe sepsis in cats include pyothorax, septic peritonitis, bacteremia secondary to GI disease, pneumonia, endocarditis, pyelonephritis, osteomyelitis, pyometra, and bite wounds.7 In cats with pyothorax, identified risk factors include indoor-outdoor environment and multicat households.215 In cats with peritonitis, neoplasia39 or trauma148 are the most commonly recognized causes. Endotoxemia is likely a component of some of these infections. Sepsis is also recognized as a cause of neonatal death in puppies and kittens. In a human study performed in a tertiary care hospital, 68% of individuals admitted to the wards or the intensive care unit had signs of SIRS. Of those patients, 26% went on develop sepsis, 18% developed sepsis with hypotension or hypoperfusion (severe sepsis), and only 4% developed septic shock. Not all patients, even those who developed septic shock, had bacteremia. Pneumonia and urinary tract infections were the most common sources of infection in patients with septic shock. Diseases associated with development of septic shock included cardiovascular disease, neoplasia, GI disease, diabetes mellitus, renal disease, respiratory disease, and pancreatitis.157 Uncomplicated SIRS-sepsis is not a negative prognostic finding if recognized early and managed appropriately; however, the prognosis worsens as an animal develops severe sepsis, septic shock, and MODS. Endotoxin is released into the intestinal lumen or tissues when gram-negative bacteria lyse, as occurs with rapid bacterial replication or with bactericidal action of some antibacterials. Within the intestinal lumen, most endotoxin is bound by bile salts and contained by the mucosal barrier; the small amounts normally absorbed into the portal circulation are cleared by hepatic macrophages. Endotoxin absorbed through the lymphatic system is cleared in regional lymph nodes. Thus, endotoxin levels in the systemic circulation are usually extremely low to immeasurable. Clinical signs of endotoxemia occur only when binding and clearance mechanisms are overwhelmed. Live bacteria can also move from the intestinal lumen through disruption of the epithelial barrier that leads to bacteremia. Bacteria and other pathogens can also gain access to the circulatory system from other mucosal surfaces, surgery, invasive monitoring, and wounds. The innate immune system is equipped with pattern recognition receptors that allow a response without prior exposure to the pathogen. These receptors are part of the TLR pathway.4,100 The first step in recognition of gram-negative bacteria is the binding of LPS to the acute-phase protein LPS binding protein (the canine molecule has been described and cloned203). This protein complexes with LPS and shuttles it to the macrophage, where the complex binds to the surface receptor, CD14, a glycosylphosphatidylinositol-anchored membrane protein that lacks a transmembrane domain. CD14 can be shed from cells, and soluble (free) CD14-endotoxin complexes can bind to endothelial cells and induce a cytokine response. It is only recently that the recognition of the TLR4 receptor provided the missing link between LPS binding to CD14 and the initiation of the signaling cascade.80 In response to the activation of the TLR4 receptor, the transcription factor nuclear factor kappa B (NF-κB; originally identified in B lymphocytes) is activated and leads to the expression of many of the proinflammatory cellular glycoproteins called cytokines.150 This activation requires the recruitment and activation of a series of adapter proteins and signaling molecules. Myeloid differentiation primary response protein 88 (MyD88) is an adapter protein found on all of the TLR. TLR4 activation and MyD88 recruitment leads to the activation of the interleukin (IL)-1 receptor-associated kinase (IRAK) family members, and tumor necrosis factor (TNF) receptor-associated factor-6 subsequent signaling pathways. Phosphorylated IRAK1 associates with TRAF6 and forms a complex with another signaling molecule, transforming growth factor β activated kinase (TAK1). NF-κB resides in the cytoplasm as a complex with inhibitory proteins of the inhibitors of NF-κB (I-κB) family. Eventually, TAK1 activation leads to phosphorylation of the I-κB kinase complex (IKK), releasing the transcription factor, NF-κB.4 This also leads to activation of the NF-κB pathway.4,100 Activation of NF-κB via phosphorylation of the inhibitory proteins (referred to as the canonical pathway of activation) can be triggered by cellular interactions with bacteria and bacterial products, viruses, parasites, and cytokines, involving activation of the TRL receptors 2, 3, 5, 6, and 9, or cytokine receptors.150 The phosphorylation step involves three IKKs and results in proteosomal degradation of the inhibitory protein. Once released from the inhibitory I-κB, NF-κB is free to translocate to the nucleus and regulate expression of numerous genes involved in the inflammatory response. NF-κB can also be activated independent of the IKK complex by hypoxia, free radicals, and ultraviolet radiation.150 Activation of NF-κB may be a biomarker of inflammation and serve as a therapeutic target for diseases of excessive inflammation. The wide range of genes regulated by NF-κB, however, may result in untoward systemic effects if the transcription factor is completely inhibited. Similar to endotoxin, products of gram-positive bacteria, fungi, and bacterial or viral DNA are capable of binding TLR receptors and activating the innate immune response. In SIRS, the innate immune system is activated by DAMPs, like the DNA binding protein, high mobility group box 1 (HMGB1).220 This highly conserved ubiquitous nuclear protein is released from cells dying from necrosis and, after acetylation or ADP ribosylation, can be actively secreted by macrophage, monocytes, and dendritic cells. HMGB1 activates TLRs 2, 4, and 9 and the receptor for advanced glycation end products. In addition, HMGB1 can enhance the response to other danger signals. Circulating HMGB1 levels persist longer than most other cytokines in acute inflammatory states, and HMGB1 may represent a therapeutic target to limit inflammation. HMGB1 induces the release of numerous cytokines from inflammatory cells, including TNF-α, IL-1 α and β, IL-1 receptor antagonist, IL-6, IL-8, and macrophage inflammatory protein-1 α and β. The macrophage is the main cell of the innate immune system that initiates the response to PAMPS and DAMPS (Web Fig. 36-1). The early macrophage response to endotoxin is characterized by release of multifunctional proinflammatory cytokines, especially IL-1β and TNF-α. In dogs, TNF-α levels increase within 15 minutes of exposure to LPS, peak at 2 hours, and return to baseline levels in 4 hours. TNF-α mediates many immune and inflammatory functions, as well as further stimulating macrophages to secrete IL-1, IL-6, macrophage colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor. IL-1 is released in similar burst fashion; it is a major co-stimulator of type 2 helper T (Th2) cells, stimulates the acute-phase response, induces fever, and has a multitude of other proinflammatory effects. Other cytokines, such as IL-6, IL-8, and interferon (IFN)-α, are becoming recognized as additional early mediators in SIRS-sepsis (see Tizard197 for further discussion of the various cytokines involved in SIRS-sepsis). IL-1, TNF-α, and so forth are released early and transiently in response to endotoxin, before the animal shows clinical signs. Novel therapeutics directed toward these mediators have not demonstrated significant benefit in most human clinical trials.113 However, late mediators of SIRS, such as HMGB1, are now being described and may be more appropriate therapeutic targets.7 The cytokine cascades increase the levels or activity (or both) of inflammatory phospholipid-derived mediators (prostacyclin, thromboxane, and platelet-activating factor),202 coagulation factors, complement, reactive oxygen species, and nitric oxide (which is an important molecule associated with hypotension),53,127,129,152 endothelin-1 (a vasoconstrictor),128 β-endorphin, histamine, serotonin, vasopressin, angiotensin II, and catecholamines. The biologic effects of these mediators include neutrophil activation, aggregation, and chemotaxis; platelet activation and aggregation; a procoagulable state; increased activity of plasma proteases; and vasodilation and potentially systemic hypotension, generalized endothelial inflammation, increased vascular permeability, and GI ulceration.121,133 Lysosomal enzymes such as elastase and chymotrypsin deplete fibronectin and activate blood clotting, triggering disseminated intravascular coagulation (DIC). There are multiple effect of sepsis on the coagulation cascade (Web Fig. 36-2).172 Sepsis promotes coagulation, inhibits anticoagulation, and impairs fibrinolysis. Early in sepsis, patients exhibit a procoagulant state which is followed by DIC.68,145 It is well known that inflammation leads to the exposure and induction of tissue factor, a potent procoagulant that complexes with factor VII(a) to activate factor IX and factor X and eventually lead to fibrin formation. Platelets are also activated by endotoxin, proinflammatory cytokines, and thrombin. Endothelial cell damage associated with sepsis results in to release of von Willebrand’s factor and further platelet aggregation. Activation of platelets leads to release of proinflammatory mediators and consumption of platelets. Clinically, septic patients frequently have low platelet counts. The three main anticoagulant protein systems, antithrombin (AT), tissue factor pathway inhibitor (TFPI), and protein C (PC), are all deranged during sepsis. Endothelial cells are actively involved in the production/activation of TFPI and PC. TFPI is secreted by and then bound to endothelial cells and inhibits the activation of factor X by tissue factor–factor VII(a) complexes. Sepsis impairs the binding of TFPI to endothelial cells and appears to decrease its effectiveness. The other anticoagulant protein that relies on normal endothelial function is PC. Healthy endothelial cells express a surface protein, thrombomodulin, which binds circulating thrombin (Fig. 36-1 and see Web Fig. 36-2). When thrombin is bound to thrombomodulin, it is not available for cleavage by fibrinogen, and the complex acts to cleave PC into active (a)PC. Endothelial cells exposed to cytokines have a reduction in thrombomodulin expression; thus, less PC can be activated. In addition, aPC is consumed in the inactivation of factor Va and factor VIIIa. Septic patients and dogs have reduced PC, which may be related to survival.46 The only clinical trial in sepsis that has shown any outcome benefit was a trial of aPC in patients with severe sepsis.19 Unfortunately, the benefits of aPC do not seem to be generalizable to wider populations.138 In addition, the expense and species specificity of the recombinant aPC preclude clinical trials in dogs. A major advance that resulted from investigations of aPC was recognition of the interaction between coagulation and inflammation. aPC inhibits neutrophil-endothelial interactions, limits proinflammatory cytokine production, blocks chemotaxis of neutrophils, and protects endothelial barrier function.138 AT is another anticoagulant protein reported to have anti-inflammatory functions (i.e., increase prostacyclin and decrease neutrophil-endothelial cell interactions). AT is reduced in sepsis through multiple mechanisms.172 Although AT therapy looked promising in experimental models of sepsis, no benefit of AT supplementation has been demonstrated in human clinical trials.1 In addition to increased activation of procoagulants and decreased anticoagulants, fibrinolysis is also impaired during sepsis. Initially, plasmin (the main fibrinolytic protein) activation is accelerated by increased release of plasminogen activators (tissue plasminogen activator and urokinase-type plasminogen activator) from the endothelial cells. This effect is short lived as cytokines induce the production and release of plasminogen activator inhibitor.172 Increased thrombin-activatable fibrinolysis inhibitor antigen was increased in dogs with sepsis and not other evaluated diseases.89a Fibrin deposition results from impairment of all components of the coagulation cascade and can contribute to tissue hypoxia and development of multiple organ dysfunction. Part of the innate immune system includes the complement system.218 Complement functions in neutrophil chemotaxis, drawing neutrophils to the sites of infection and inflammation, enhancing clearance of bacteria through opsonization, and causing lysis of cells (both bacterial and host). Complement is also involved in the integration between the innate and adaptive immune system and facilitates removal of apoptotic cells and immune complexes. Complement can be activated by one of three pathways, all of which converge with the activation of C3. Cleavage of C3 leads to production of the anaphylatoxin C3a and the eventual cleavage and activation of C5. C5 cleavage produces the chemotactic protein and anaphylatoxin C5a and C5b, a major component of the membrane attack complex responsible for lysing cells. The classic pathway is activated by immune complexes on cells, apoptotic cells, and some bacteria and viruses. The mannose binding lectin pathway provides an antibody-independent mechanism to activate the complement cascade by binding bacterial mannose residues. Independent of antibody binding, endotoxin and other surface molecules on bacteria activate the alternate pathway.65,218 In sepsis, high levels of C3a and C5a are associated with a worse prognosis. In addition to its role as a chemotactic protein, C5a activates endothelial cells, causes vasodilation, and increases vascular permeability. Like many inflammatory mediators, the effects of C5a appear to be complex. C5 can be proinflammatory or protective or even lead to immune paralysis. The response, at least in part, appears to depend on the amount of C5a, the receptor it binds, and the presence of proinflammatory or anti-inflammatory cytokines.64 Strategies to limit C5 activation continue to be investigated. Despite normalization of macrohemodynamic indices (blood pressure, perfusion, and global tissue oxygenation), multiorgan dysfunction and failure may still develop in septic patients. Advances in the ability to study the microcirculation have found alterations in blood flow through the arterioles, capillaries, and venules.* These changes appear to have significant prognostic implications in people with severe sepsis.200 Researchers have found that even though the percentage of perfused small vessels and vascular density were similar at the onset of shock in both survivors and nonsurvivors, small vessel perfusion improved with time only in the survivors.201 However, both survivors and nonsurvivors had similar measures of global perfusion and cardiovascular parameters after initial stabilization, demonstrating that microcirculation, not macrocirculation, is important for determining prognosis and survival. This may provide a valuable monitoring tool in veterinary patients to help guide diagnostic and therapeutic decisions and monitoring. Concurrent with inflammatory mediator upregulation, anti-inflammatory mediators are secreted to balance and limit the inflammatory response. An insufficient or inappropriate anti-inflammatory response can allow sepsis to progress.121 The prevailing theory has been that sepsis is a result of a cytokine storm, leading to uncontrolled systemic inflammation. Newer studies indicate that, for at least some patients, the hyperinflammatory state is followed by severe acute immunosuppression, caused by the apoptosis of B cells, CD4+ T cells, and follicular dendritic cells. The mechanism of immunosuppression is not known; however, release of endogenous glucocorticoids may be an important contributor.83 Thyroid hormone production and secretion have been impaired in endotoxin-treated dogs.146 In summary, PAMPs act as a signal to the immune system that pathogens have invaded the host, whereas DAMPs are signals of damage to host cells (see Web Fig. 36-1). A localized response along with a primed or controlled systemic response is beneficial in containing and resolving the infection, whereas an uncontrolled host response leads to systemic inflammation, immunosuppression, severe sepsis, septic shock, and ultimately organ failure. The clinical features of sepsis are associated with the systemic inflammatory response described previously. The diagnosis of sepsis is often presumptive in clinical practice, based on the accumulation of clinicopathologic and biochemical data. Although a “sepsis test” does not exist, it is possible to use the patient’s clinical picture combined with a high level of suspicion to quite accurately diagnose a septic patient. However, it has been reported that 82% and 60% of bacteremic dogs and cats, respectively, were found to suffer from clinical sepsis in one retrospective study.73 The most common hematologic abnormalities in people with sepsis include anemia, leukocytosis, thrombocytopenia, and activation of the hemostatic system.23 Similar changes have been reported in dogs and cats.25,39,45,175,223 A leukocytosis with increased number of bands (left shift) and cytologic evidence of toxic neutrophils indicate ongoing and premature release of leukocytes from the bone marrow and continued inflammation.175 Increased vascular endothelial permeability due to vasculitis, platelet sequestration in the lymphoreticular system, and DIC can all contribute to thrombocytopenia. Blood loss (e.g., GI loss secondary to poor perfusion and ulcerations), hemolysis, and/or decreased erythrocyte production may all lead to anemia (more common in septic cats than dogs). Potential causes of anemia in septic animals include premature clearance by the reticuloendothelial system with a low-grade hemolysis and increased susceptibility to oxidative damage leading to a Heinz body anemia, especially in cats.34,35,223,224 Hemostatic derangements, referred to as DIC, are common in humans and animals suffering from sepsis.2,45,89a,97,145 Hemostatic derangements are typically characterized by hypercoagulability initially, then hypocoagulability as the disease progresses. It is often challenging to diagnose hypercoagulability clinically; therefore, this stage is often overlooked. Some potential clues might include a progressively decreasing platelet count and fibrinogen levels, low AT activity, elevated D-dimers and fibrin/fibrinogen degradation products (FDPs), the presence of schistocytes on blood smear evaluation, evidence of hypercoagulability using thromboelastographic indices, or clinical evidence of abnormal clotting (e.g., catheters, anticoagulated blood samples, vascular thromboses to major organs). The later hypocoagulable state is more frequently recognized clinically and typifies the progression of a septic patient into DIC.45,145 A statistically significant lower protein C and AT activity and higher prothrombin time, partial thromboplastin time, D-dimer, and FDPs compared to controls have been described.45 Dogs with naturally occurring CPV enteritis had decreased AT activity and increased maximum amplitude on the thromboelastogram, consistent with hypercoagulability.145 Hematologic and hemostatic changes in septic animals are summarized in Table 36-4. TABLE 36-4 Hematologic and Hemostatic Changes in Sepsis FDP, Fibrinogen degradation products; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell count. aLess commonly performed tests; may only be available through reference laboratories. From Boller EM, Otto CM. 2009. Sepsis, pp 454-458. In Silverstein DC, Hopper K (eds): Small animal critical care medicine. Elsevier, St Louis. Biochemical profile changes in animals with sepsis are typically reflective of the underlying disease process (e.g., azotemia with pyelonephritis), but may also be due to sepsis-induced changes (e.g., cholestasis). Patients with severe sepsis and septic shock often develop derangements indicative of organ dysfunction or failure (e.g., hepatic failure, DIC). Some of the more common, nonspecific abnormalities include hyperglycemia hypoglycemia, hypoalbuminemia, and hyperbilirubinemia.25,45,135,177,193 The early detection of sepsis is desirable because mortality increases with the development of organ dysfunction, hypotension, and hyperlactatemia. Early clinical signs such as tachypnea, tachycardia, fever, and leukocytosis are nonspecific, so research investigating the use of biomarkers is ongoing. Biomarkers are generally biochemical or cellular measures of a biologic state or process. Ideal biomarkers of sepsis would be able to not only detect the presence of an infection, but also temporally track the severity of the infection and progression from sepsis to severe sepsis and septic shock. Their ability to monitor response to treatment and provide prognostic information is also desirable. Potential categories of early diagnostic biomarkers might include markers of infection (e.g., LPS, bacterial DNA), markers of cellular responsiveness (intercellular cellular adhesion molecule 1, CD11/CD18),226 and products of inflammatory cells and humoral activation (ILs, AT, aPC).45 Procalcitonin and neutrophil CD64 appear to be potentially useful biomarkers for human sepsis, but their application to veterinary patients is yet to be defined.79,81,118,139 Heparin binding protein has been found to be more predictive of septic shock in people compared to several other well-studied biomarkers.101 Additional biomarkers that have received attention in people include IL-6, aPC (also known as activated drotrecogin alfa), acute-phase proteins (C-reactive protein, amyloid A, haptoglobin, fibrinogen, α-1-acid glycoprotein, and ceruloplasmin), LPS, LPS-binding protein, nitrate/nitrite concentrations (by-products of nitric oxide metabolism), soluble intercellular cellular adhesion molecule 1, AT, TFPI, CD14, CD11b/CD18, T-cell function assays, IL-12, neutrophil gelatinase-associated lipocalin, and IL-1 receptor antagonist.29,30,178,214 Serum IL-6 has been studied in experimental models of endotoxemia or inflammation in dogs; this biomarker rises within hours, stays elevated for days when inflammation persists, and may have a prognostic role in this species.130,131,158,170 Initial serum C-reactive protein concentration in dogs with sepsis did not correlate with survival rate.68a,229a However, decreasing concentrations were observed with recovery from sepsis. Monocyte chemoattractant protein-1 is increased in critically ill dogs and highest levels have been observed in those with sepsis.56a This level of cytokine mediator in dogs appeared to increase in proportion to the severity of sepsis, similar to what has been observed in humans. aPC is decreased in septic human patients and canine models of sepsis and represents a therapeutic target that reduces mortality in some populations with severe sepsis.17,106,106 However, treatment with aPC is quite costly and species specific; therefore, clinical use of this product is limited in veterinary medicine. Plasma von Willebrand factor antigen concentration, a marker of entothelial activation, was increased in dogs with sepsis as compared to that in clinically healthy control dogs.163a However, there was no significant difference between levels in surviving and non-surviving dogs.
Sepsis
Etiology
Term
Definition
Bacteremia
Living bacterial organisms in circulating blood
Endotoxemia
Lipopolysaccharide of gram-negative bacterial cell wall circulating in blood
Systemic inflammatory response syndrome
Systemic signs of inflammation (two of the four criteria)
Sepsis
Confirmed infection plus systemic signs of inflammation
Severe sepsis
Sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion
Sepsis-induced hypotension
Systolic blood pressure (SBP) <90 mmHg, mean arterial pressure <70 mmHg, or a decrease in SBP of >40 mmHg
Septic shock
Sepsis-induced hypotension despite adequate fluid resuscitation
Sepsis-induced hypoperfusion
Septic shock with an elevated lactate or oliguria
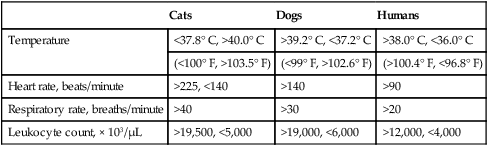
Cats
Dogs
Humans
Temperature
<37.8° C, >40.0° C
>39.2° C, <37.2° C
>38.0° C, <36.0° C
(<100° F, >103.5° F)
(<99° F, >102.6° F)
(>100.4° F, <96.8° F)
Heart rate, beats/minute
>225, <140
>140
>90
Respiratory rate, breaths/minute
>40
>30
>20
Leukocyte count, × 103/µL
>19,500, <5,000
>19,000, <6,000
>12,000, <4,000
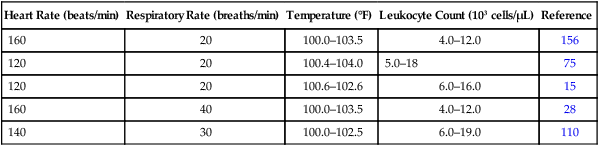
Heart Rate (beats/min)
Respiratory Rate (breaths/min)
Temperature (°F)
Leukocyte Count (103 cells/µL)
Reference
160
20
100.0–103.5
4.0–12.0
156
120
20
100.4–104.0
5.0–18
75
120
20
100.6–102.6
6.0–16.0
15
160
40
100.0–103.5
4.0–12.0
28
140
30
100.0–102.5
6.0–19.0
110
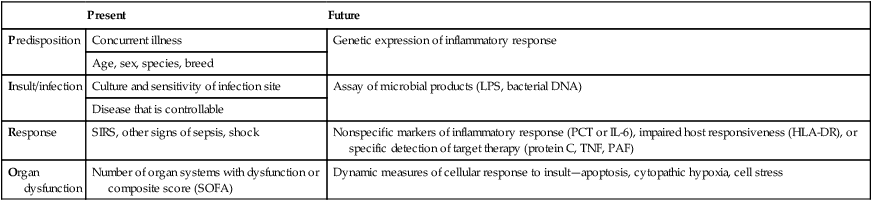
Present
Future
Predisposition
Concurrent illness
Genetic expression of inflammatory response
Age, sex, species, breed
Insult/infection
Culture and sensitivity of infection site
Assay of microbial products (LPS, bacterial DNA)
Disease that is controllable
Response
SIRS, other signs of sepsis, shock
Nonspecific markers of inflammatory response (PCT or IL-6), impaired host responsiveness (HLA-DR), or specific detection of target therapy (protein C, TNF, PAF)
Organ dysfunction
Number of organ systems with dysfunction or composite score (SOFA)
Dynamic measures of cellular response to insult—apoptosis, cytopathic hypoxia, cell stress
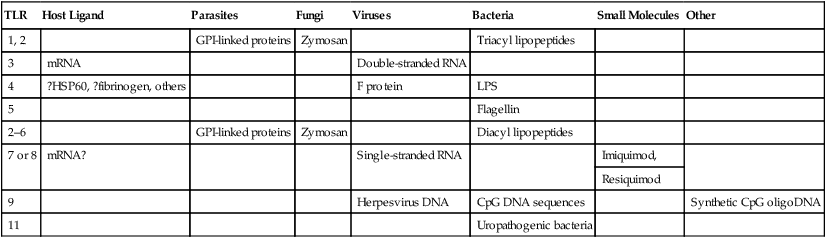
TLR
Host Ligand
Parasites
Fungi
Viruses
Bacteria
Small Molecules
Other
1, 2
GPI-linked proteins
Zymosan
Triacyl lipopeptides
3
mRNA
Double-stranded RNA
4
?HSP60, ?fibrinogen, others
F protein
LPS
5
Flagellin
2–6
GPI-linked proteins
Zymosan
Diacyl lipopeptides
7 or 8
mRNA?
Single-stranded RNA
Imiquimod,
Resiquimod
9
Herpesvirus DNA
CpG DNA sequences
Synthetic CpG oligoDNA
11
Uropathogenic bacteria
Epidemiology
Pathogenesis
Clinical Signs
Diagnosis
Clinical Laboratory Findings
Hematologic Findings
Coagulation Abnormalities
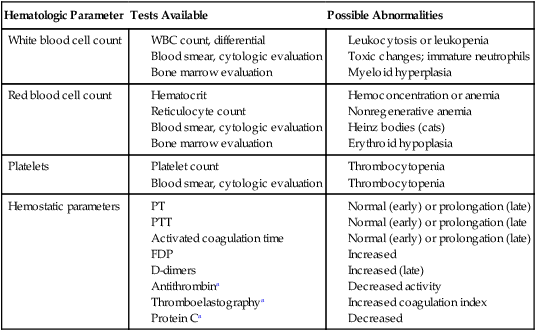
Hematologic Parameter
Tests Available
Possible Abnormalities
White blood cell count
Red blood cell count
Platelets
Hemostatic parameters
Serum Biochemical Findings
Biomarkers
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sepsis