CHAPTER 5 Respiratory Tract
Cytologic evaluation of the respiratory tract in correlation with history, clinical data, and imaging results provides invaluable diagnostic information that directly impacts patient management. Cytologic features seen in the respiratory tract following injury, disease, and primary or metastatic neoplasia depend largely on the normal underlying structure and function of the cellular elements. Thorough examination of a high-quality sample is critical for obtaining meaningful cytology results. This chapter describes appropriate sampling techniques and cytologic interpretation of samples from the respiratory tract, including the nasal cavity, larynx, airways, and lung parenchyma.
THE NASAL CAVITY
Normal Anatomy and Histologic Features
The vomeronasal organ is bilaterally symmetric and located along the base of the nasal septum in the rostral part of the nasal cavity. The organ is comprised of various components including epithelium, ducts, glands, and connective tissue (Salazar et al., 1996) and, at least in dogs, is also rich in tissue of neuronal origin (Dennis et al., 2003). In addition to neuron cell bodies and axon fascicles, the sensory epithelium also expresses neuronal markers.
Collection Techniques and Sample Preparation
When history and clinical signs suggest disease of the nasal cavity, the first diagnostic step is a thorough inspection of the external and internal nasal cavity, pharynx, hard and soft palates, and oral cavity including examination of the gingiva and upper dental arcade for oronasal fistulation and periodontal disease. Additionally, palpation for enlarged regional lymph nodes and subsequent aspiration and/or biopsy may provide a valuable indirect means of achieving a diagnosis if disseminated or metastatic disease is present. Magnetic resonance imaging (MRI) and computed tomography (CT) provide additional data regarding the extent of the lesion, airway involvement, and three-dimensional localization (e.g., of foreign bodies). However, in most cases radiographs remain a reliable tool to localize mass lesions for diagnostic sampling (Jones and Ober, 2007; Petite and Dennis, 2006) although they are less sensitive for differentiating inflammatory and neoplastic rhinitis (Kuehn, 2006). Adequate visual inspection of the nasal cavity endoscopically and localization of lesions via radiographs or other imaging techniques will enable the appropriate collection techniques to be employed. However, it should be noted that rhinoscopic assessment does not uniformly predict the presence or absence of inflammatory disease; thus obtaining a sample for microscopic evaluation is critical (Johnson et al., 2004; Windsor et al., 2004). Examination by flexible endoscope is preferred as approximately 50% to 80% of the nasal cavity cannot be visualized through examinations by either a rigid endoscope or an otoscope (Elie and Sabo, 2006). If an endoscope is unavailable, an otoscope may be used to examine the rostral nasal cavity, and with aid of a dental mirror and light, a portion of the nasopharynx can also be visualized. Radiography and rhinoscopy should be performed prior to sampling as hemorrhage may hinder radiographic interpretation and obscure visualization during endoscopy.
Nasal Swabs
The presence of an acute or chronic nasal discharge indicates upper respiratory disease but is nonspecific and may be present with inflammatory, infectious, or neoplastic disorders. Nasal discharge may be unilateral or bilateral and range from serous, suppurative, mucoid, to serosanguineous depending on the underlying cause(s). Superficial and deep nasal swabs are easy to obtain and relatively nontraumatic, but often do not provide much information beyond identifying superficial inflammation, secondary bacterial infection, hemorrhage, necrosis, and mucus, while the underlying disease process remains obscure. As a general rule, invasive techniques allowing collection of tissue deep to the nasal mucosa increase diagnostic potential. For example, successful detection of aspergillosis increases from positive detection of 13% to 20% of samples examined by a direct smear or blinds swab to 93% to 100% positive detection in samples obtained by brush cytology or biopsy (De Lorenzi et al., 2006a). However, occasionally the simplest technique can be rewarding, such as the cytologic examination of a nasal swab for the diagnosis of cryptococcosis infection in cats. Therefore, cytologic examination of nasal exudate should be performed initially in any nasal disease.
Nasal Flush
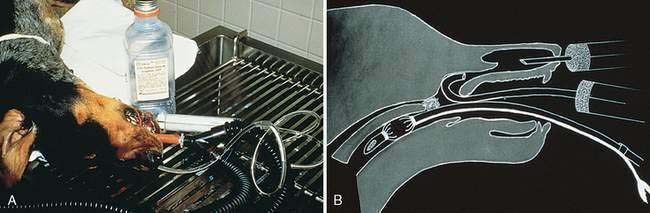
Nasal flushing methods have been reviewed elsewhere (Smallwood and Zenoble, 1993). In general, invasive and aggressive techniques are more likely to yield diagnostic material. Nontraumatic nasal flushes only produce material for a definitive diagnosis in approximately 50% of the cases. A 6 to 10 French polypropylene or soft red rubber urinary catheter is inserted into the external nares to flush sterile, nonbacteriostatic, physiologic saline or lactated Ringer’s solution through the nasal cavity (Fig. 5-1A). A traumatic nasal flush can be accomplished by beveling or nicking the tubing or catheter, creating a rough surface to aid in dislodging tissue. As with any instrument placed into the nasal cavity, penetration through the cribiform plate into the cranial vault can be avoided by measuring the distance from the external nares to the medial canthus of the eye and cutting the tubing or catheter to the appropriate length, or marking the instrument with tape.
Small aliquots (5 to 10 ml) of fluid are introduced into the nasal cavity via a 20- to 35-ml syringe with alternating positive and negative pressure. As the fluid enters the cavity, the tubing or catheter is aggressively moved back and forth against the nasal turbinates in an attempt to free tissue fragments that can be collected on gauze sponges held below the external nares or reaspirated into the collection syringe. An alternative method involves directing a Foley catheter into the oral cavity and retroflexing around the soft palate into the nasopharynx, inflating the bulb, and lavaging the saline so that the fluid passes through the nasal cavity and out the external nares for collection (Fig. 5-1B).
Imprint and Brush Cytology
Alligator biopsy forceps are used to obtain a pinch biopsy for impression cytology and histopathology, while an endoscopic brush is used to collect tissue to roll on a glass slide for cytology. Both sampling techniques are typically performed with endoscopic guidance. Imprint cytology can also be performed on core biopsy samples obtained using a Tru-Cut Disposable Biopsy Needle (Cardinal Health Allegiance, Deerfield, IL). Similarly, the polypropylene portion of an indwelling catheter with the needle removed, or a polypropylene urinary catheter with the end cut at a 45-degree angle can also be used to obtain tissue specimens. The catheter is pushed into the mass and rotated while applying negative pressure. Tissue can then be rolled on a glass slide or used to make touch imprints for cytologic evaluation before placing in 10% neutral buffered formalin. Brush cytology often misses the deeper inflammatory cells and may not correlate well with histologic results (Michiels et al., 2003). Therefore, deeper, more invasive samples are preferred wherever possible. In one study of 54 dogs with nasal tumors, brush and imprint cytology correctly identified neoplasia of epithelial origin in 88% and 90% of the cases, respectively (Clercx et al., 1996). However, in the same study, the ability to diagnose mesenchymal tumors was significantly less. Histologic diagnosis correlated with 50% of imprint cytology impressions and only 20% of those made by brush cytology.
Normal Cytology and Common Cytologic Changes
Normal Nasal Cytology
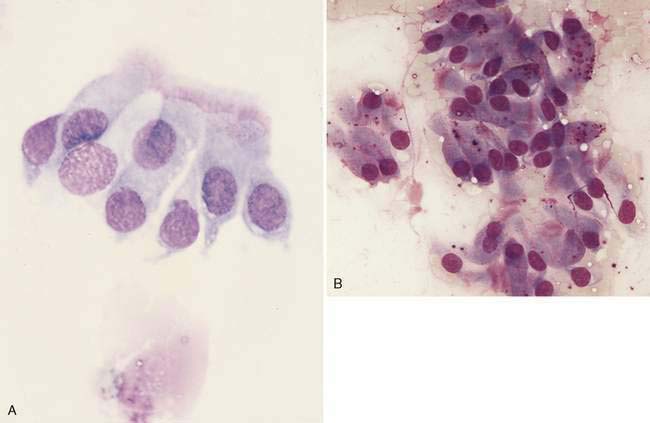
Nasal swabs and flushes of healthy animals contain few cells, small amounts of mucus, and low numbers of a mixed population of extracellular bacteria (normal flora) found colonizing the surface of epithelial cells. Ciliated columnar respiratory epithelial cells from the posterior nasal cavity typically predominate; however, small numbers of squamous epithelial cells originating from the anterior nasal cavity may also be present. Respiratory epithelial cells can be seen singly or in small clusters, are columnar and contain a round, basally located nucleus. Cilia, if present, are located opposite the nucleus and can be seen as an eosinophilic brush border (Fig. 5-2A). While also columnar with a basally located nucleus, goblet cells lack cilia, are more plump, and contain a moderate amount of cytoplasm with numerous prominent, round, purple-staining cytoplasmic mucin granules (Fig. 5-2B). Occasionally, basal epithelial cells may be seen. When present, these cells are round to cuboidal with scant, deeply basophilic cytoplasm and round, centrally placed nuclei. On cytologic specimens, mucus appears as an eosinophilic amorphous extracellular material that often entraps cells. Nasal-associated lymphoid tissue (NALT) can be found in the nasal cavity of both the dog and the cat and responds similarly to organized lymphoid tissue in other location (Fig. 5-3). The canine and feline nasal cavity contains NALT and lymphoid follicles, particularly in the nasopharynx. These islands of lymphocytes can respond similarly to other organized lymphoid tissue such as lymph nodes. The degree of hemorrhage observed is contingent on the collection procedure. Erythrocytes with platelet clumps and white blood cells in numbers and proportions consistent with blood (approximately one white cell per 500 to 1000 red cells) indicate iatrogenic contamination of the sample or peracute hemorrhage. The nasal cavity of the dog and cat harbor large numbers of a mixed population of both aerobic and anaerobic bacteria that are considered normal microflora including Streptococcus sp., Staphylococcus sp., Escherichia coli, Pseudomonas sp., Proteus sp., and Bordetella bronchiseptica.
Oropharyngeal Contamination
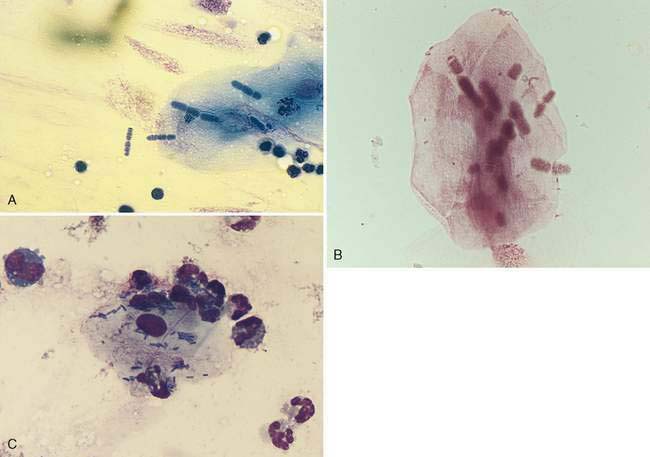
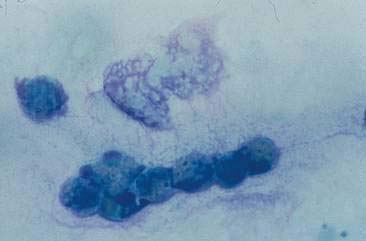
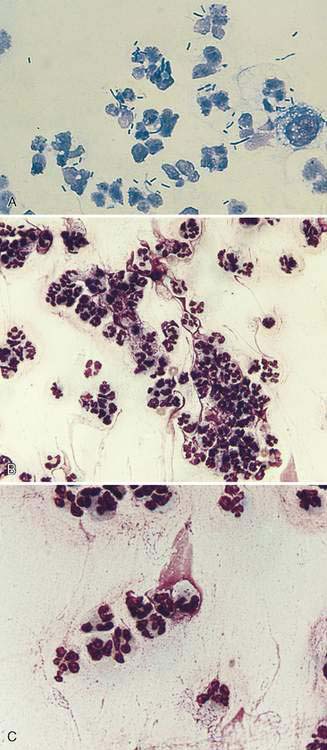
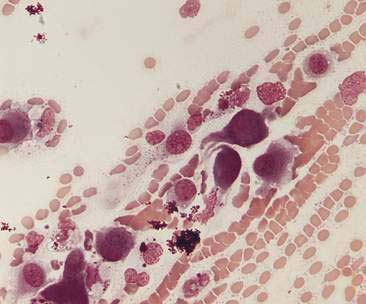
Oropharyngeal contamination is seen most frequently in samples collected by flushing techniques. The presence of Simonsiella sp. is a hallmark of oropharyngeal contamination. Simonsiella sp. are large, rod-shaped bacteria that align in a row after division resulting in a distinctive pattern that resembles stacked coins (Fig. 5-4A&B). Oropharyngeal contamination is also characterized by the presence of a mixed population of bacteria found extracellularly that colonize the surface of keratinized squamous epithelial cells. If oropharyngeal inflammation is present (e.g., periodontal disease), inflammatory cells may be seen associated with the oropharyngeal contamination (Fig. 5-4C).
Hyperplasia/Dysplasia
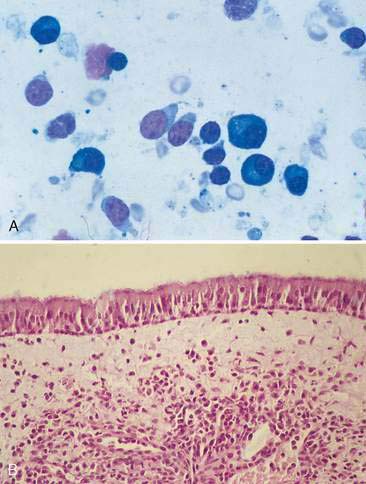
Chronic inflammation secondary to various infectious and noninfectious etiologies (e.g., trauma, chronic irritation, or neoplasia) is common in the nasal cavity and can have a profound effect on the integrity and function of normal cellular constituents. Several adaptive mechanisms are employed by cells to survive amid the inflammatory stimulus. Increased numbers of cells, or hyperplasia, is one such mechanism and is often accompanied by dysplasia (Fig. 5-5A). Dysplasia is readily identified histologically as a loss of architectural organization but is more difficult to identify in cytologic preparations, which typically lack structural features. Samples from an inflamed nasal cavity with hyperplasia and dysplasia are likely to contain numerous clusters and sheets of epithelial cells with an increased nuclear-to-cytoplasm ratio, mild to moderate anisocytosis, and increased cytoplasmic basophilia (Fig. 5-5B). Mitotic figures, while normal in appearance, may be increased as well. Hyperplasia and dysplasia are reversible but may represent early neoplastic changes and can be difficult to differentiate cytologically from well-differentiated carcinoma.
Metaplasia
Another adaptive response to chronic irritation/inflammation is metaplasia. Metaplasia involves a change in cellular differentiation such that a susceptible specialized normal cell type is transformed to one that is better able to endure the environmental stress while losing specialized function. In the respiratory system, metaplasia is often characterized by the transformation of columnar respiratory epithelial cells to a more squamous phenotype resulting in a loss of the ability to produce and secrete protective mucus. Cytologically, squamous metaplasia is detected by the presence of squamous epithelial cells either as the primary cell type or admixed with more normal respiratory epithelial cells (French, 1987). Cells may be present in sheets or individually depending on the degree of keratinization. Basilar cells tend to remain in clusters, while more keratinized squamous cells often appear individually and have angular borders, abundant hyalinized, basophilic cytoplasm and small, and occasionally pyknotic or karyorrhectic nuclei. As with hyperplasia, neoplastic transformation of the squamous cells may occur.
Nasal melanosis has also been suggested as a metaplastic transformation of the nasal respiratory mucosa and has been reported rarely in dogs with odontopathic rhinitis (De Lorenzi et al., 2006b).
Noninfectious Inflammatory Disease
Allergic Rhinitis
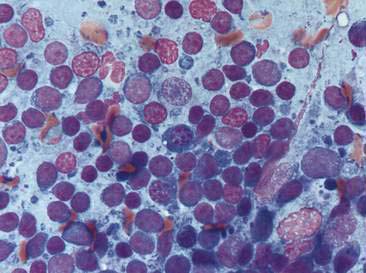
Hypersensitivity may occur in the nasal cavity alone or concurrent with involvement of the lower airways. The inflammatory infiltrate associated with an allergic rhinitis is characterized predominantly by eosinophils, with lesser numbers of neutrophils, occasional mast cells, and occasional plasma cells (Fig. 5-6). Increased numbers of goblet cells and abundant mucus may also be seen along with rafts of hyperplastic respiratory epithelial cells. Differentials for eosinophilic inflammation include parasitic and fungal infection. Mast cell tumors should also be considered; however, the numbers of mast cells often helps to differentiate these two processes. Typically, mast cells comprise only a small proportion of the inflammatory infiltrate in allergic rhinitis, whereas mast cells tend to be the predominant cell present in mast cell tumors.
Lymphoplasmacytic Rhinitis
Until recently, only occasional cases of idiopathic lymphoplasmacytic rhinitis had been described (Burgener et al., 1987; Tasker et al., 1999b). This chronic nasal disease in dogs was thought to be immune mediated rather than allergic in origin. However, a more recent study has identified that idiopathic lymphoplasmacytic rhinitis may be more common than previously suspected (Windsor et al., 2004) and may be associated with, and even contribute to, chronic nasal disease in dogs, ultimately resulting in turbinate remodeling and even bony destruction. Despite histologic evidence of bilateral disease in most dogs, a unilateral discharge was seen in some of the cases indicating the need to examine both sides of the nasal cavity even in cases that appear localized in origin. Lack of response to glucocorticoid therapy in the latter report (Windsor et al., 2004) suggests mechanisms other than immune-mediated disease, although multifactorial disease including an immune-mediated component cannot be excluded. Other proposed etiologies include immune dysregulation, allergies, or disruption of the normal microbial flora. Because aspergillosis can also induce a lymphoplasmacytic rhinitis (see Fig. 5-3), there has been concern that idiopathic lymphoplasmacytic rhinitis represents occult aspergillosis. However, analysis of cytokine profiles from nasal biopsies from dogs with these two diseases indicates that the immunologic pattern is quite different. Aspergillosis induces a predominantly T-helper type 1 (Th1) response while a partial Th2 response was detected in cases of idiopathic lymphoplasmacytic rhinitis (Peeters et al., 2007). This type 2 response is in contrast to the type 1 cytokine profile reported in cats with chronic inflammation of the nasal cavity (Johnson et al., 2005), which suggests different underlying etiologies and pathogenesis between these species.
Nasal Polyps
Nasal polyps are occasionally reported in dogs but occur most commonly in cats and are characterized by hyperplasia of the mucous membranes or exuberant proliferation of fibrous connective tissue. Polyps originate within the nasopharyngeal region from the Eustachian tube, middle ear, or nasopharynx. The majority of affected cats are young, often less than 1 year of age (Moore and Ogilvie, 2001). Nasal polyps are speculated to develop secondary to chronic irritation. Although occasional reports have suggested an underlying infectious etiology, the cause of nasal polyps remains unclear in most cases. Inflammatory polyps have the same epithelial and/or connective tissue hyperplasia but also contain a prominent inflammatory infiltrate. Polyps appear grossly as small, smooth, well-circumscribed, pedunculated masses arising from the mucosal surface of the nasal cavity. Clinical signs are usually apparent when the polyp enlarges enough to occlude the nasopharynx. Cytologically, mature lymphocytes and plasma cells are often admixed with rafts of epithelial cells (Fig. 5-7A). Small numbers of neutrophils and macrophages may also be present. Squamous metaplasia and/or dysplasia are frequently seen and, when present, can make the distinction from epithelial neoplasia difficult.
Chronic Sinusitis
Recurrent clinical signs of sneezing and nasal congestion may be related to infectious agents, parasites, allergies, foreign bodies, or neoplasia. A cause may not be demonstrated on cytology or histology in some of these cases. Cytologically, respiratory epithelium appears reactive as evidenced by hyperplasia, dysplasia, or metaplasia. Inflammatory infiltrates often consist of mixed mononuclear cells, including small to medium-sized lymphocytes, plasma cells, and macrophages (Fig. 5-7B).
Infectious Causes
Bacteria
With the exception of Bordetella bronchiseptica and Pasturella multocida, which may cause acute rhinitis in the dog, primary bacterial rhinitis is rare. However, secondary bacterial infection is common and may accompany nasal neoplasia, viral infection, fungal infection, parasitic infection, trauma, foreign bodies, dental disease, or oronasal fistulation (Fig. 5-8). Infection with Mycoplasma sp. and Chlamydia sp. in cats may cause mild upper respiratory signs concurrently with conjunctivitis.
Primary bacterial infection of the nasal cavity is determined cytologically by finding large numbers of a primarily monomorphic bacteria accompanied by a marked suppurative inflammatory response with numerous phagocytized bacteria (Fig. 5-9A). Mucus may be abundant and can obscure identification of bacteria in some cases (Fig. 5-9B&C). Culture of the nasal exudate reveals heavy growth of one type of organism. However, a uniform population of organisms can also be detected with secondary or opportunistic pathogens. Therefore, because bacterial rhinitis is uncommon, significant effort should be made to identify any possible underlying causes. PCR may also be useful for detection of certain organisms, such as Mycoplasma sp. Identification of the bacteria as bacilli or cocci may aid initial institution of antimicrobial therapy as cocci are typically gram-positive and bacilli are typically gram-negative. The presence of filamentous organisms forming mats of colonies suggests Actinomyces and Nocardia spp. Regardless, culture and sensitivity are necessary for proper identification of microorganisms. In addition, whether bacteria are present as a primary or secondary pathogen or as an asymptomatic inhabitant of the nasal cavity, antimicrobial resistance may be of concern.
Fungal
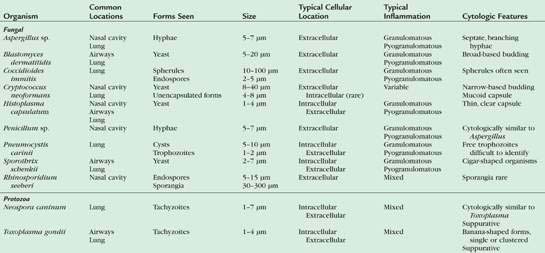
The diagnosis of fungal rhinitis can be complicated because while fungal infection can be a primary disease, fungal organisms may also be found as secondary and opportunistic invaders. In addition, fungi such as Aspergillus sp. and Penicillium sp. can occasionally be cultured from the nasal cavity of clinically normal dogs. Aspergillus sp. and Penicillium sp. are the most common fungal agents in mycotic rhinitis in the dog, whereas Cryptococcus sp. occurs most frequently in cats. Upper respiratory involvement with Histoplasma capsulatum and Blastomyces dermatitidis has also been reported but is rare (Table 5-1).
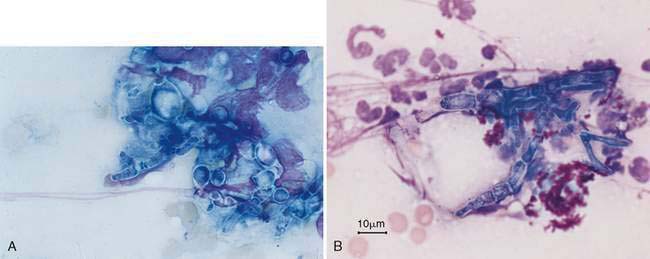
Infection with Aspergillus sp. can be associated with purulent, granulomatous, or pyogranulomatous inflammation. Infection and inflammation may be present in the nasal cavity alone, the frontal sinus alone, or both sites (Johnson et al., 2006). Cytologically, fungal hyphae are branching, septate, 5-7μm in width, with straight, parallel walls and globose terminal ends. Hyphae can stain either intensely basophilic with a thin, clear outer cell wall or appear as negatively staining images against a cellular background (Fig. 5-10). Hyphae may be difficult to identify when found in low numbers or in dense mats admixed with mucus, inflammatory cells, and cellular debris. Occasionally, round to ovoid blue-green fungal spores may also be observed. Periodic acid-Schiff or silver stains (GMS) are useful when fungal organisms are suspected but not readily identified in the sample. While cytologic characteristics may suggest aspergillosis, culture is necessary for a definitive diagnosis. Similar to bacterial rhinitis, the presence of fungal elements does not rule out underlying neoplasia or other disorders.
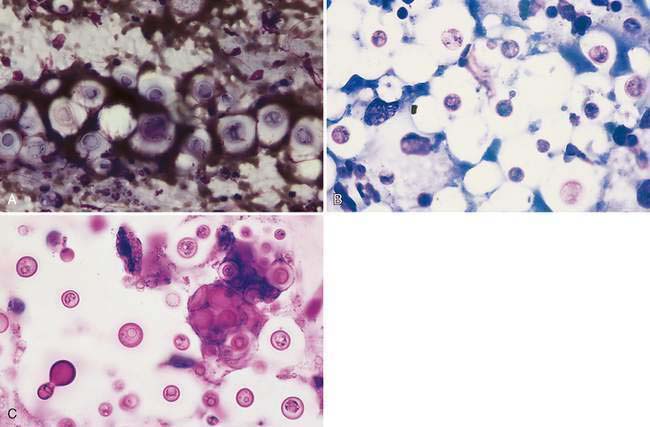
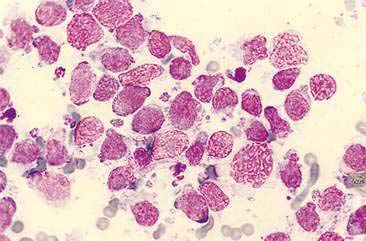
Cryptococcus sp. is a common cause of chronic upper respiratory disease in cats and is also occasionally reported in dogs. However, both Cryptococcus neoformans and Cryptococcus gattii have been reported in the nasal passages of dogs and cats in the absence of local or systemic infection (Duncan et al., 2005; Malik et al., 1997), suggesting that subclinical infection or asymptomatic carriage needs to be considered when the organism is detected in an otherwise normal animal. Inhalation is the suspected route of infection. Concurrent ocular, cutaneous, or neurologic disease may also be seen in animals with Cryptococcus rhinitis. Immunity is speculated to play a role in the development of infections as well as in dissemination of infection throughout the body. Corticosteroid therapy during infection worsens the symptoms as well as the disease progression (Greene, 1998; Medleau and Barsanti, 1990). However, underlying diseases, in particular those that are immunosuppressive (e.g., FeLV, FIV), have not been proven to be predisposing factors to infection (Flatland et al., 1996; Medleau and Barsanti, 1990). Organisms are readily identified in swabs of nasal exudates or imprints/aspirates from nasal masses (Fig. 5-11A&B). Positive identification of the organism via cytology is diagnostic; however, serologic testing and fungal culture may also be useful. New methylene blue (Fig. 5-11C) and India ink can be used to demonstrate the negative staining capsule; however, care must be taken not to mistake air bubbles and fat droplets for organisms. Cryptococcus sp. are round to oval yeast that range in diameter from 8 to 40 μm (including the capsule). The organism has a granular internal structure that stains eosinophilic to purple and is surrounded by a thick, nonstaining, mucoid capsule. The capsule material can give the sample a mucinous texture. Occasionally, narrow-based budding may be seen. Unencapsulated or rough forms are 4 to 8 μm and are difficult to distinguish from H. capsulatum. Fungal culture and serology are useful in this case. The presence and type of inflammation range from the observation of a few to no inflammatory cells amid a field full of organisms to pyogranulomatous inflammation. The degree and type of inflammation may be related to characteristics of the capsule.
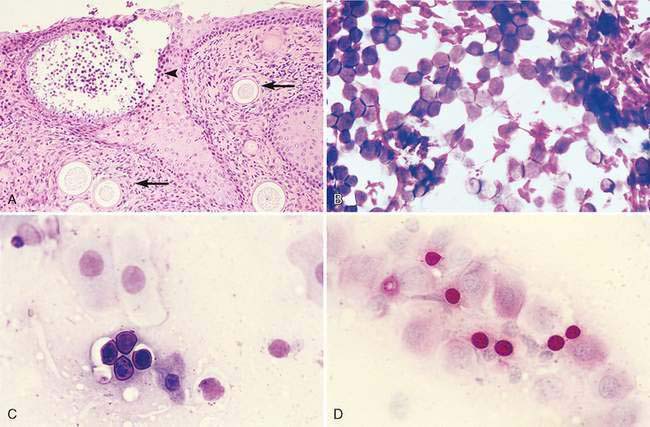
Rhinosporidium seeberi occasionally infects the nasal cavity of dogs resulting in single to multiple polyps in which numerous small, miliary sporangia can be observed on the surface. The pathogenesis of infection is unclear, but contact with water and trauma to the nasal mucous membranes are predisposing factors. Cytologically, preparations contain variable numbers of magenta-staining spores that range in diameter from 5 to 15μm. They have slightly refractile capsules and contain numerous, round, eosinophilic structures (spherules). In some cases, the spores stain deeply eosinophilic, preventing visualization of the internal structures. Sporangia are variably sized. They are often very large (range 30 to 300μm) (Fig. 5-12A), well-defined, globoid structures that undergo sporulation to contain numerous small, round endospores (Fig. 5-12B&C). Sporangia are not commonly observed in stained smears because the wall of the sporangia are slightly refractile and do not stain. Sporangia can be observed in unstained direct preparations (Caniatti et al., 1998). Endospores within the sporangia are brown when observed microscopically before staining and appear as three different basophilic forms or stages of maturation with Romanowsky stains (Meier et al., 2006). Immature endospores are approximately 2 to 4 μm in diameter with lightly basophilic cytoplasm and a pink-purple nucleus encompassing ⅓ to ½ of the endospore and 1 to 2 smaller round magenta structures. Intermediate endospores are rarely described but appear to be spherical, granular, basophilic structures approximately 5 to 8 μm in diameter with eosinophilic to globular internal structures and a variably sized, clear halo. Mature endospores tend to predominate in cytologic preparations. These structures are 8 to 15 μm in diameter, with a thick, hyalinized cell wall and a pale, magenta to nonstaining halo. Internal structure can be difficult to visualize in thick areas of the prep, but when endospores are spread out, numerous small spherical eosinophilic globular internal structures can be seen. PAS staining enhances the chance of finding the spores in cytologic or histologic specimens (Fig. 5-12D). Rhinosporidiosis incites a mixed inflammatory response consisting of neutrophils, plasma cells, and lymphocytes. Macrophages, mast cells, and eosinophils are less commonly observed. Rosetting of inflammatory cells, particularly neutrophils, around the spores has been observed and is considered a useful feature in finding the spores during cytologic examination under low magnification (Gori and Scasso, 1994).
Sporotrichosis has been rarely identified in samples from the nasal cavity of dogs (Cafarchia et al., 2007; Whittemore and Webb, 2007). The paucity of organisms and cytologic appearance is similar to that reported for Sporothrix schenckii from other canine samples. Sporothrix schenckii has also been isolated in the nasal cavity of cats with sporotrichosis and is more commonly detected in those with cutaneous lesions (Leme et al., 2007).
Nasal mycosis due to infection of cats by Alternaria species, one of the dematiaceous fungi that induce phaeohyphomycosis, has been recently reported in three cats from the United Kingdom (McKay et al., 2001; Tennant et al., 2004). Cytologic findings include the presence of pyogranulomatous inflammation, lymphocytes, and plasma cells. Fungal organisms are pale staining, oval to round with septate hyphae of approximately 7 to 14 μm having a narrow peripheral clear area and finely stippled eosinophilic internal material.
Parasitic
Parasitic rhinitis is uncommon in dogs and cats and may or may not be associated with clinical signs (King et al., 1990). Infection with Eucoleus aerophilus (formerly Capillaria aerophila) is diagnosed by finding the adult nematodes or characteristic ova in nasal secretions. The ova are large (60 × 35 μm), ovoid in shape, with two asymmetrical terminal plugs. Mixed inflammation often containing eosinophils is present. The nasal cavity and frontal sinuses of dogs may be inhabited by several forms of the arthropod parasite Linguatula serrata. Because the ova are infrequently seen in nasal exudates, this parasite is most readily diagnosed by direct visualization via rhinoscopy. The ova measure 90 × 70μm, larvae up to 500 μm, and nymphs measure 4 to 6 mm. Infection with this parasite most commonly elicits mild signs such as sneezing and nasal discharge, but occasionally severe clinical signs occur. The nasal mite Pneumonyssoides caninum is best diagnosed by direct rhinoscopic visualization of the off-white, 1- to 2-mm adult mites inhabiting the nasal cavity and paranasal sinuses of dogs. Infection results in a mild, transient rhinitis.
Protozoal
Leishmania sp. may induce masses in the nasal cavity of dogs. Amastigotes can be identified in aspirates or biopsies from dogs with leishmaniasis (Llanos-Cuentas et al., 1999).
Algal
Prototheca sp. may produce a nasal mass in cats near the nares resulting from a cutaneous infection. Cytologically, aspirate or swab preparations reveal a mixture of inflammatory cells, mostly degenerate neutrophils and macrophages along with numerous sporulated and nonsporulated endospores. The endospores present as variably sized spheres having a thin, clear rim and a granular, dense center (see Fig 3-23A&B).
Neoplasia of the Nasal Cavity and Paranasal Sinuses
Tumors can arise from any of the numerous tissue types found in the nasal cavity and paranasal sinuses (Table 5-2). Identification of the site of origin can be difficult, as most malignant tumors are locally invasive, destructive, and have extended into surrounding tissues by the time of diagnosis. The majority of tumors involve the caudal two thirds of the nasal cavity near or adjacent to the cribiform plate. Less commonly, tumors may be located in the paranasal sinuses. Malignant neoplasia often involves the nasal turbinates and septum and can extend through the maxilla into the oral cavity. Extension into the orbit and cranial vault via erosion through the cribiform plate is less common but does occur. Metastasis to regional lymph nodes tends to occur late in the disease and is most often associated with epithelial tumors.
TABLE 5-2 Neoplasia of the Nasal Cavity
| Tissue of Origin | Benign Neoplasia | Malignant Neoplasia |
|---|---|---|
| Epithelial | ||
| Mesenchymal | ||
| Round cell (discrete) |
* Indicates most common tumor types.
Epithelial Neoplasia
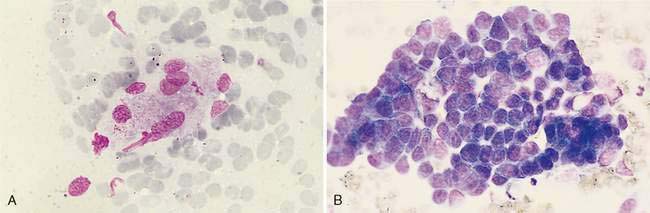
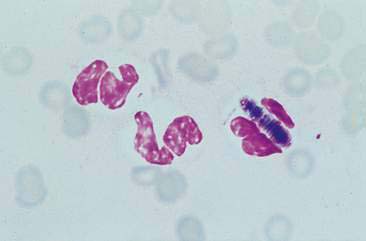
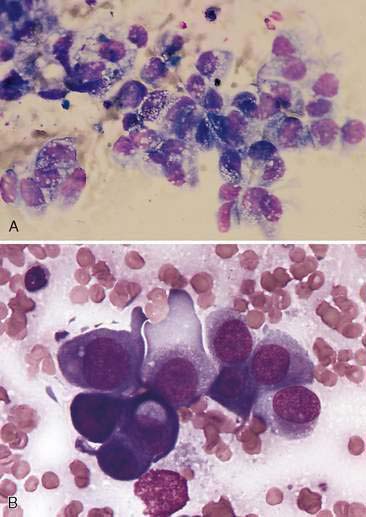
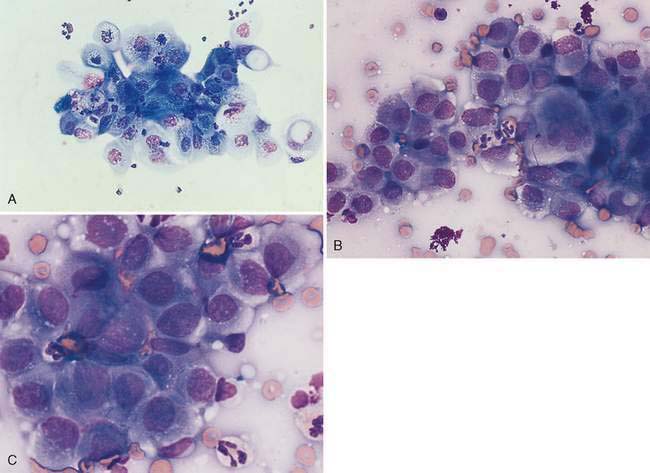
Malignant epithelial tumors of the nasal cavity occur more frequently than their benign counterparts. The most common epithelial tumors of the nasal cavity include adenocarcinomas, squamous cell carcinomas, transitional carcinomas, and anaplastic or undifferentiated carcinomas. Adenocarcinomas are common in dogs and cats, while SCC are more common in cats (Carswell and Williams, 2007). Cytologic samples from carcinomas tend to be moderately cellular. Neoplastic epithelial cells are present in small aggregates to larger sheets (Fig. 5-13A&B). Adenocarcinomas can be identified by the presence of ring or rosette acinar arrangements that are best visualized at low magnification (e.g., 10×) (Fig. 5-14). Malignant epithelial cells are round to polygonal and typically display numerous criteria of malignancy. Such features include macrocytosis, moderate to marked anisocytosis, anisokaryosis, an increased nuclear-to-cytoplasm ratio, and deeply basophilic cytoplasm that may contain numerous discrete, clear cytoplasmic vacuoles or one large, clear vacuole (signet ring form) suggestive of secretory product. Nucleolar criteria of malignancy should also be assessed, evaluating for the number of nucleoli per nucleus and any size or shape variations. Anaplastic cells may individualize and appear similar to lymphoid cells but large cell size and periodic sheet formation are helpful in distinguishing the two types of neoplasms (Fig. 5-15A&B). Extracellular secretory material such as mucus may also be identified as eosinophilic, amorphous to fibrillar material.
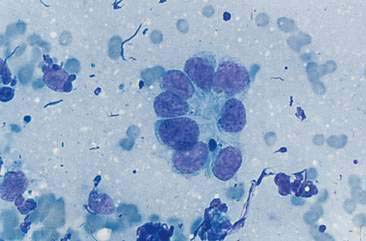
SCC are distinguished by the presence of cells with angular borders containing abundant, homogenous, glassy cytoplasm and centrally placed nuclei. The neoplastic cells display a wide range in maturation, ranging from immature, small, cuboidal, nucleated, epithelial cells with deeply basophilic cytoplasm to more mature cells, identified as anucleate, fully keratinized cells containing abundant, pale, basophilic cytoplasm and sharply angulated borders. Evidence of asynchronous development may be present such as the identification of fully keratinized cells with retained large nuclei (Fig. 5-16A). Prominent anisokaryosis and variable chromatin patterns ranging from smooth (immature) to clumped (mature) may be seen. A few neoplastic squamous cells may also show a perinuclear clearing (perinuclear “halo”) or even a few, small, clear, punctate, perinuclear vacuoles. Abundant keratinaceous debris represented as amorphous, basophilic extracellular material is often scattered about the slides. A common characteristic of SCC is the presence of a moderate to marked accompanying neutrophilic inflammatory response.
Similar in cytologic appearance to SCC is a neoplasm termed transitional carcinoma. This neoplasm arises from nonciliated nasal respiratory epithelium (Carswell and Williams, 2007). It may display a moderately abundant cytoplasm with numerous punctate vacuoles. Malignant features often involve anisokaryosis, multinucleation, coarse chromatin clumping, prominent nucleolus, and variable nuclear-to-cytoplasmic ratios (Fig. 5-16B&C).
Neuroendocrine Carcinoma
Neuroendocrine carcinomas or carcinoids have been rarely described in the nasal cavity of dogs (Patnaik et al., 2002; Sako et al., 2005). Histologically their features appear to be similar to those described elsewhere in the body. Cytologic characteristics have not been reported.
Mesenchymal Neoplasia
Mesenchymal neoplasia of the nasal cavity is uncommon. However, when present, osteosarcoma, fibrosarcoma, and chondrosarcoma are the mesenchymal tumors most often seen (Fig. 5-17). When present, chondrosarcomas are more likely to occur in young dogs with a possible increased risk in medium to large breeds (Lana and Withrow, 2001). Cytologic samples are typically of low cellularity consisting of individualized and occasionally small, loose aggregates of oval, plump, or spindle-shaped cells (Figs. 5-18A&B). Cytoplasmic borders are typically ill defined and neoplastic cells may contain few to moderate numbers of fine eosinophilic to purple cytoplasmic granules. Matrix may be observed as streaming, brightly eosinophilic, fibrillar material often intimately associated with the neoplastic cells. However, this material is easily confused with mucus, and the presence or absence of streaming eosinophilic material on a cytologic preparation should not be used to differentiate the type of neoplasia.
A cytologic diagnosis of mesenchymal neoplasia is complicated by several factors. Mesenchymal neoplasia often exfoliates poorly resulting in a hemodiluted sample that contains only a few pleomorphic spindle-shaped cells for evaluation. Also, significant inflammation can induce reactive fibroplasia that can be difficult to distinguish from fibrosarcoma. In this case, cytologic evaluation coupled with physical exam and historical and radiographic information raises the index of suspicion for mesenchymal neoplasia, warranting biopsy with histopathologic examination for definitive diagnosis. Additionally, histopathology is often necessary for classification of mesenchymal neoplasia, as the more commonly seen mesenchymal tumors often lack distinguishing cytologic features. Other types of mesenchymal tumors involving the upper airways (see Table 5-2) are uncommon but have cytologic features resembling soft tissue sarcomas in more common sites.
Discrete Cell Neoplasia
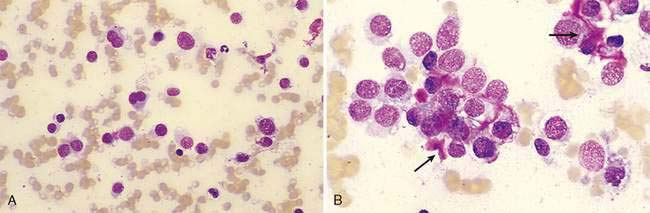
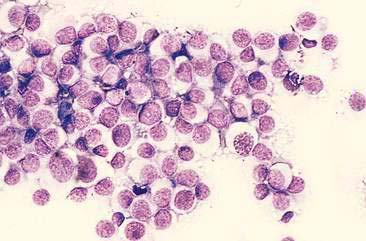
Lymphoma is the most common discrete cell tumor reported in the nasal cavity of dogs and cats. In cats, the majority of nasal lymphomas are of B-cell origin, although T-cell lymphoma of the nasal cavity has also been reported (Day et al., 2004; Mukaratirwa et al., 2001). Lymphoma of the nasal cavity tends to be characterized by a monomorphic population of medium-sized or large, immature lymphoblasts with scant, deeply basophilic cytoplasm, large round nuclei, finely granular chromatin, and single to multiple nucleoli (Fig. 5-19). Anaplastic nasal carcinomas (see Fig. 5-15A) can individualize and resemble lymphoma but the presence of very large cells and occasional sheet formation will assist in making the proper diagnosis. Care should be taken to distinguish lymphoma from lymphoid hyperplasia or an inflammatory polyp (see Fig. 5-7A). In lymphoid hyperplasia, a heterogeneous population of lymphocytes and plasma cells are present, with a predominance of small, mature lymphocytes and fewer intermediate-sized lymphocytes and lymphoblasts. In some cases, lymphoma is characterized by a predominance of intermediate-sized lymphocytes with an increased amount of cytoplasm and smooth chromatin lacking nucleoli. Even more problematic are cases where the neoplastic population consists of small, well-differentiated lymphocytes. In such questionable cases, histopathology is imperative for definitive diagnosis of lymphoma.
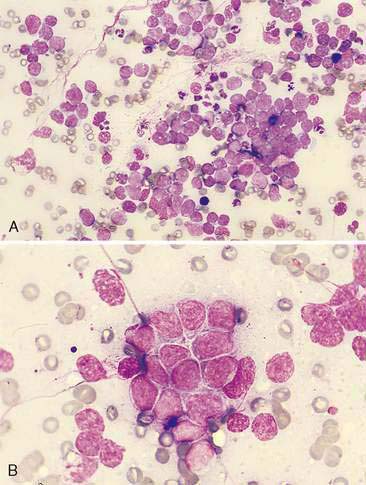
Canine transmissible venereal tumor (TVT) is a contagious neoplasm involving the external genitalia of both sexes with a low occurrence of metastasis. Spread to the nasal cavity is thought to occur secondary to implantation from a primary genital tumor; however, there are several reports of primary intranasal TVT (Ginel et al., 1995; Papazoglou et al., 2001; Perez et al., 1994). Cytologic preparations reveal large numbers of a monomorphic population of large, round cells with abundant, light to moderately basophilic cytoplasm containing numerous, distinct, small vacuoles. Nuclei are round with coarse to ropy chromatin with 1 or 2 large, prominent nucleoli. Mitoses are frequently observed (Fig. 5-20).
Histiocytic Sarcoma
Canine histiocytic neoplasia can present as either a local or disseminated process. Localized histiocytic sarcomas tend to arise from the subcutis but occasionally originate from other sites including the nasal cavity (Affolter and Moore, 2002). The morphology of cells in this report varied from site to site, as well as within different nodules of the same tumor; however, it was similar in phenotype and variation to those previously described.
Miscellaneous Neoplasia
Oncocytoma of the nasal cavity has been rarely reported in dogs and cats (Doughty et al., 2006) (see section on laryngeal oncocytoma for discussion of cytologic features).
Intranasal and sinus melanoma appears to be uncommon, but has been reported in both dogs (Hicks and Fidel, 2006) and cats (Mukaratirwa et al., 2001).
LARYNX
Sample Collection
Respiratory stridor, dyspnea, and changes in or loss of vocal tone suggest laryngeal disease. Cytologic evaluation of the larynx is most useful for the characterization of mass lesions, infiltrative processes, or inflammatory disease and depends on obtaining adequate, representative samples. Laryngeal masses, while uncommon, may be detected and stabilized for sampling by palpation. Radiographs may help detect and localize mass lesions, but may be difficult to interpret due to breed variations and superimposition of soft tissues. Ultrasonographic evaluation affords superior visualization of laryngeal masses and guidance for FNA. Ultrasound-guided aspiration through the ventral laryngeal cartilage has not been associated with significant complications, even in cats (Rudorf and Brown, 1998).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree