CHAPTER 9 To facilitate the understanding of the structure and function, it is convenient to arbitrarily divide the respiratory system into conducting, transitional, and gas exchange systems (Fig. 9-1). The conducting system includes nostrils, nasal cavity, paranasal sinuses, nasopharynx, larynx, trachea, and extrapulmonary and intrapulmonary bronchi, all of which are largely lined by pseudostratified, ciliated columnar cells plus a variable proportion of secretory goblet (mucous) and serous cells (Figs. 9-2 and 9-3 and Web Fig. 9-1). The transitional system of the respiratory tract is composed of bronchioles, which serve as a transition zone between the conducting system (ciliated) and the gas exchange (alveolar) system (see Fig. 9-1). The disappearance of cilia in the transitional system is not abrupt; the ciliated cells in the proximal bronchiolar region become scarce and progressively attenuated, until the point where distal bronchioles no longer have ciliated cells. Normal bronchioles also lack goblet cells, but instead have other types of secretory cells, notably Clara and neuroendocrine cells. Clara cells, also referred to as secretory bronchiolar cells, contain numerous biosynthetic organelles that play an active role in detoxification of xenobiotics (foreign substances), similar to the role of hepatocytes (Fig. 9-4). Clara cells are also critical stem cells in the repair and remodeling of not only the bronchioles, but of most of the respiratory tract. In addition, Clara cells contribute to the innate immunity of the lung by secreting protective proteins (collectins) and pulmonary surfactant (Fig. 9-4, B). In carnivores and monkeys, and to a much lesser extent in horses and humans, the terminal portions of bronchioles are not only lined by cuboidal epithelium but also by segments of alveolar capillaries. These unique bronchioloalveolar structures are known as respiratory bronchioles (Fig. 9-5; also see Fig. 9-1). The gas exchange system of the respiratory tract in all mammals is formed by alveolar ducts and millions of alveoli (Fig. 9-6; also see Fig. 9-1). The surface of the alveoli is lined by two distinct types of epithelial cells known as type I pneumonocytes (membranous) and type II pneumonocytes (granular) (Fig. 9-7). Fig. 9-1 Schematic diagram of airways from the trachea to the alveoli. Fig. 9-2 Normal mucosa, trachea dog. Fig. 9-3 Schematic representation of the mucociliary apparatus of the conducting system. Fig. 9-4 Normal bronchiole, rat. Fig. 9-5 Normal respiratory bronchiole, dog. Fig. 9-6 Lung, rat. Fig. 9-7 The blood-air barrier. All three—the conducting, transitional, and exchange systems of the respiratory system—are vulnerable to injury because of constant exposure to a myriad of microbes, particles and fibers, and toxic gases and vapors present in the air. Vulnerability of the respiratory system to aerogenous (airborne) injury is primarily because of (1) the extensive area of the alveoli, which are the interface between the blood in alveolar capillaries and inspired air; (2) the large volume of air passing continuously into the lungs; and (3) the high concentration of noxious elements that can be present in the air (Table 9-1). For humans, it has been estimated that the surface of the pulmonary alveoli is approximately 200 m2, roughly the area of a tennis court. It has also been estimated that the volume of air reaching the human lung every day is around 9000 L. The surface of the equine lung is estimated to be around 2000 m2. TABLE 9-1 Common Pathogens, Allergens, and Toxic Substances Present in Inhaled Air Microbes, toxins, and pneumotoxicants can gain access into the respiratory system by the following routes (also see Tables 9-1 and 9-2): TABLE 9-2 Portals of Entry into the Respiratory System 1. Aerogenous—Pathogens, such as bacteria, mycoplasmas, and viruses, along with toxic gases and foreign particles, including food, can gain access to the respiratory system via inspired air. This is the most common route in the transmission of most respiratory infections in domestic animals. 2. Hematogenous—Some viruses, bacteria, parasites, and toxins can enter the respiratory system via the circulating blood. This portal of entry is commonly seen in septicemias, bacteremias, and protozoa and viruses that target endothelial cells. Also, circulating leukocytes may release infectious organisms such as retroviruses and Listeria monocytogenes while traveling through the lungs. 3. Direct extension—In some instances, pathogenic organisms can also reach the pleura and lungs through penetrating injuries, such as gunshot wounds, migrating awns, or bites, or by direct extension from a ruptured esophagus or perforated diaphragm. It is axiomatic that a particle, microbe, or toxic gas must first gain entry to a vulnerable region of the respiratory system before it can induce an adaptive immune response or have a pathologic effect. The characteristics of size, shape, dispersal, and deposition of particles present in inspired air are studied in aerobiology. It is important to recognize the difference between deposition, clearance, and retention of inhaled particles. Deposition is the process by which particles of various sizes and shapes are trapped within specific regions of the respiratory tract. Clearance is the process by which deposited particles are destroyed, neutralized, or removed from the mucosal surfaces. The difference between what is deposited and what is cleared from the respiratory tract is referred to as retention. The main mechanisms involved in clearance are sneezing, coughing, mucociliary transport, and phagocytosis (Table 9-3). Abnormal retention of particles resulting from increased deposition, decreased clearance, or a combination of both is the underlying pathogenetic mechanism in many pulmonary diseases (Fig. 9-8). TABLE 9-3 Main Defense Mechanisms of the Respiratory System Fig. 9-8 Pulmonary clearance and retention of bacteria following inhalation of an experimental aerosol of bacteria. The anatomic configuration of the nasal cavity and bronchi plays a unique role in preventing or reducing the penetration of noxious material into the lungs, especially into the alveoli, which is the most vulnerable portion of the respiratory system. The narrow nasal meatuses and the coiled arrangement of the nasal conchae generate enormous turbulences of airflow and as a result, physical forces are created that forcefully impact particles larger than 10 µm onto the surface of the nasal mucosa (Fig. 9-9). Although particles smaller than 10 µm could escape trapping in the nasal cavity, these medium-sized particles meet a second barrier at the tracheal and bronchial bifurcations. Here, abrupt changes in the direction of air (inertia), which occurs at the branching of major airways, cause particles in the 2- to 10-µm size range to collide with the surface of bronchial mucosa (see Fig. 9-1). Because the velocity of inspired air at the level of the small bronchi and bronchioles has become rather slow, inertial and centrifugal forces no longer play a significant role in the trapping of inhaled particles. Here, in the transitional (bronchiolar) and exchange (alveolar) regions, particles 2 µm or smaller may come into contact with the mucosa by means of sedimentation because of gravitation or by diffusion as a result of Brownian movement. Infective aerosols containing bacteria and viruses are within the size ranges (0.01 to 2 µm) that typically gain access to the bronchioloalveolar region. Fig. 9-9 Dorsal (D), ventral (V), and ethmoidal (E) conchae, midsagittal section of head, cow. Mucociliary clearance is the physical unidirectional movement and removal of deposited particles and gases dissolved in the mucus from the respiratory tract. Mucociliary clearance, also referred to as the waste disposal system, is provided by the mucociliary blanket (mucociliary escalator) and is the main defense mechanism of the conducting system (nasal cavity, trachea, and bronchi) (see Figs. 9-2 and 9-3). Mucus acts primarily as a barrier and a vehicle and is a complex mixture of water, glycoproteins, immunoglobulins, lipids, and electrolytes produced by goblet (mucous) cells, serous cells, submucosal glands, and fluid from transepithelial ion and water transport. Once serous fluid and mucus are secreted onto the surface of the respiratory mucosa, a thin, double-layer film of mucus is formed on top of the cells. The outer layer of this film is in a viscous gel phase, whereas the inner layer, which is in a fluid or sol phase, is directly in contact with cilia (see Fig. 9-3). A healthy human produces around 100 mL of mucus per day. Each ciliated cell in the conducting system has around 100 to 200 motile and chemosensory cilia (6 µm long), beating metachronously (forming a wave) at a ciliary beat frequency of approximately 1000 strokes per minute, and in a horse, for example, mucus moves longitudinally at a rate of up to 20 mm per minute. Rapid and powerful movement of cilia creates a series of waves that, in a continuous and synchronized manner, propel the mucus, exfoliated cells, and entrapped particles out of the respiratory tract to the pharynx. The mucus is finally swallowed, or when present in large amounts, it is coughed up out of the conducting system. If mucus flow were to move at the same rate in all levels of a conducting system, a “bottleneck” effect would be created in major airways as the minor but more numerous airways enter the bronchi. For this reason, the mucociliary transport in proximal (rostral) airways is physiologically faster than that of the distal (caudal) ones. Ciliary activity and mucus transport increase notably in response to stimuli such as in respiratory infections. Alveoli lack ciliated and mucus-producing cells; thus the defense mechanism against inhaled particles in the alveolar region cannot be provided by mucociliary clearance. Instead, the main defense mechanism of alveoli (exchange system) is phagocytosis provided by the pulmonary alveolar macrophages (Fig. 9-10). These highly phagocytic cells, which are not to be confused with intravascular pulmonary macrophages, are derived largely from blood monocytes and to a much lesser extent, from a slowly dividing population of interstitial macrophages. After a temporary adaptive stage within alveolar interstitium, blood monocytes reduce their glycolytic metabolism and increase their oxidative metabolism to function in an aerobic rather than an anaerobic environment. Pulmonary alveolar macrophages contribute to the pulmonary innate and adaptive immune response rapidly attaching and phagocytosing bacteria and any other particle reaching the alveolar lumens. The number of free macrophages in the alveolar space is closely related to the number of inhaled particles reaching the lungs. This ability to increase, within hours, the number of available phagocytic cells is vital in protecting the distal lungs against foreign material, particularly when the inhaled particle load is high. Unlike that of tissue macrophages, the lifespan of alveolar macrophages in the alveoli is notably short, only a few days, and thus they are continuously being replaced by newly migrated blood monocytes. Fig. 9-10 Pulmonary alveolar macrophages. Alveolar phagocytosis plays a prominent role in the innate defense mechanism against inhaled bacteria without the need of an inflammatory reaction. Bacteria reaching the alveoli are rapidly phagocytosed, and bactericidal enzymes present in lysosomes are discharged into the phagosome containing the bacteria (see Fig. 9-10). Except for some facultative pathogens that are resistant to intracellular killing (e.g., Mycobacterium tuberculosis, Listeria monocytogenes, Brucella abortus, and some Salmonella spp.), most bacteria reaching the lungs are rapidly destroyed by activated alveolar macrophages. Similarly, inhaled particles, such as dust, pollen, spores, carbon, or erythrocytes from intraalveolar hemorrhage, are all phagocytosed and eventually removed from alveoli by pulmonary alveolar macrophages. Most alveolar macrophages leave the alveoli by migrating toward the bronchiolar (transitional) region until the mucociliary blanket is reached. Once there, pulmonary macrophages are removed in the same way as any other particle: along the mucociliary flow to the pharynx and swallowed. In the cat, as many as 1 million macrophages per hour move out from the alveoli into the conducting system and pharynx. Destruction and removal of inhaled microbes and particles by alveolar macrophages is a well-orchestrated mechanism that engages many cells, receptors (i.e., Toll-like receptors [TLRs]) and pulmonary secretions in the lung. The cell-to-cell interactions are complex and involve pulmonary alveolar macrophages, pneumonocytes, endothelial cells, lymphocytes, plasma cells, natural killer (NK) cells, and dendritic cells. Antibodies are also important in the protection (acquired immune response) of the respiratory tract against inhaled pathogens. IgA is the most abundant antibody in the nasal and tracheal secretions and prevents the attachment and absorption of antigens (immune exclusion). IgG and to a lesser extent IgE and IgM promote the uptake and destruction of inhaled pathogens by phagocytic cells (immune elimination). IgG is the most abundant antibody in the alveolar surface and acts primarily as an opsonizing antibody for alveolar macrophages and neutrophils. In addition to antibodies, there are several secretory products locally released into the alveoli that constitute the alveolar lining material and contribute to the pulmonary defense mechanisms. The most important of these antimicrobial products are transferrin, anionic peptides, and pulmonary surfactant (Table 9-4). TABLE 9-4 Defense Mechanisms Provided by Some Cells and Secretory Products Present in the Respiratory System *Superoxide dismutase, catalase, glutathione peroxidase, oxidant free radical scavengers (tocopherol, ascorbic acid). Lungs are also susceptible to hematogenously borne microbes, toxins, or emboli. The hepatic (Kupffer cells) and splenic macrophages are the primary phagocytic cells responsible for removing circulating bacteria and other particles from the blood of dogs, some rodents, and humans. In contrast, the cell responsible for the removal of circulating particles, bacteria, and endotoxin from the blood of ruminants, cats, pigs, and horses is mainly the pulmonary intravascular macrophage, a distinct population of phagocytes normally residing within the pulmonary capillaries (see Fig. 9-7). In pigs, 16% of the pulmonary capillary surface is lined by pulmonary intravascular macrophages. In ruminants, 95% of intravenously injected tracer particles or bacteria are rapidly phagocytosed by these intravascular macrophages. Recent studies showed that an abnormally reduced number of Kupffer cells in diseased liver results in a compensatory increase in pulmonary intravascular macrophages, even in animal species in which these phagocytic cells are normally absent from the lung. In some abnormal conditions, such as sepsis, excessive release of cytokines by pulmonary intravascular macrophages may result in acute lung injury. Viral agents are notorious in predisposing humans and animals to secondary bacterial pneumonias by what is known as viral-bacterial synergism. A good example of this synergistic effect of combined virus-bacterial infections is documented from epidemics of humans with influenza virus in which the mortality rate has been significantly increased from secondary bacterial pneumonia. The most common viruses incriminated in predisposing animals to secondary bacterial pneumonia include influenza virus in pigs and horses; bovine herpesvirus 1 (BoHV-1), parainfluenza-3 (PI-3), and bovine respiratory syncytial virus (BRSV) in cattle; canine distemper virus in dogs; and herpesvirus and calicivirus in cats. The mechanism of the synergistic effect of viral-bacterial infections was previously believed to be the destruction of the mucociliary blanket and a concurrent reduction of mucociliary clearance, but in experimental studies, viral infections did not significantly reduce the physical removal of particles or bacteria out of the lungs. Now, it is known that 5 to 7 days after a viral infection, the mucociliary clearance and phagocytic function of pulmonary alveolar macrophages are notably impaired (see Fig. 9-8). Other mechanisms by which viruses impair defense mechanisms are multiple and remain poorly understood (Box 9-1). Immunization against viral infections in many cases prevents or reduces the synergistic effect of viruses and thus the incidence of secondary bacterial pneumonia Web Fig. 9-1 Ultrastructural morphology of respiratory mucosa. The lungs should be examined before incision. Normal lungs typically have a homogeneous pink color (Web Fig. 9-2). External changes include the presence of rib imprints on the pleural surface when lungs fail to collapse. In addition, the lungs should be inspected for changes in color and texture and distribution of lesions. Color changes can be various shades of red, indicating hypostatic congestion, hyperemia (acute pneumonia), and hemorrhage; dark blue collapsed lobules or areas are indicative of atelectasis; pale pink to white lungs indicate notable anemia, fibrosis, or emphysema; and uniformly or patchy yellow-brown lungs indicate chronic passive congestion and pulmonary fibrosis likely secondary to chronic heart failure. Lungs from exsanguinated animals are generally paler than the normal pink color because of reduced blood in the pulmonary tissue. A covering of yellowish material on the pleural surface indicates accumulation of fibrin. Because it is impossible to describe the texture of normal lungs, experience in palpation is required to appreciate the actual texture of a normal lung. Texture is determined by gently palpating the surface and parenchyma of the lungs. Normal texture can change to firm, hard, elastic (rubbery), or crepitus (with a crackling sound or feeling). For a detailed description of lung texture, see the section on Classification of Pneumonias. Palpation of the lungs, which should be gentle, also permits detection of nonvisible nodules or abscesses in the parenchyma. Knowing the distribution of a lesion in the lungs also facilitates diagnosis because particular etiologic agents cause lesions with specific distribution. Distribution of lesions is generally described as focal, multifocal, locally extensive, or diffuse. According to their topography, pulmonary lesions can also be classified as cranioventral, dorsocaudal, and so on. Web Fig. 9-2 Normal lung, pig. Two valuable diagnostic tools in human medicine, bronchoalveolar lavage (BAL) and tracheal aspirates, have in recent years become more widely used in veterinary clinical diagnosis of respiratory ailments, particularly in horses, dogs, and cats. The basis of BAL is sampling to determine the cellular and biochemical composition of the lung in a respiratory patient live animal by infusing and retrieving sterile fluid via the trachea. BAL is done by inserting a tube directly through the larynx into a bronchus, or transtracheally by inserting a tube through a needle percutaneously into the cervical trachea. Microscopic examination of properly collected, stored, and processed samples may reveal many erythrocytes and siderophages in pulmonary hemorrhage or left-sided heart failure; inclusion bodies or syncytial cells in viral pneumonias; increased number of leukocytes in pulmonary inflammation; abundant mucus in asthma or equine heaves (chronic obstructive pulmonary disease [COPD]); presence of pulmonary pathogens, such as parasites, fungi, and bacteria; or tumor cells in cases of pulmonary neoplasia. In the healthy patient, 80% to 95% of the BAL cells are pulmonary alveolar macrophages (see Fig. 9-10). Pseudostratified ciliated epithelium, which lines most of the nasal cavity and nasopharynx, part of the larynx, and all of the trachea and bronchi, is exquisitely sensitive to injury. When these cells are irreversibly injured, whether caused by a viral infection, trauma, or inhalation of toxic gases, ciliated cells swell, typically lose their attachment to underlying basement membrane, and rapidly exfoliate (Fig. 9-11). A transient and mild exudate of fluid, plasma proteins, and neutrophils covers the ulcer. In the absence of complications or secondary bacterial infections, a specific type of progenitor cell known as nonciliated secretory cells, which is normally present in the mucosa, migrates to cover the denuded basement membrane and undergoes mitosis, eventually differentiating into new ciliated epithelial cells (see Fig. 9-11). Cellular migration, proliferation, and attachment are regulated by locally released growth factors and extracellular matrix (ECM) proteins such as collagen, integrins, and fibronectin. The capacity of ciliated epithelium to repair itself is remarkably effective. For example, epithelial healing in an uncomplicated ulcer of the tracheal mucosa can be completed in only 10 days. This sequence of cell degeneration, exfoliation, ulceration, mitosis, and repair is typically present in many viral infections in which viruses replicate in nasal, tracheal, and bronchial epithelium, causing extensive mucosal ulceration. Examples of transient infections of this type include human colds (rhinoviruses), infectious bovine rhinotracheitis (bovine herpesvirus 1), feline rhinotracheitis (feline herpesvirus 1), and canine infectious tracheobronchitis (CAV-2 and canine parainfluenza-2). Fig. 9-11 Normal and injured nasal epithelium following exposure to air containing an irritant gas (hydrogen sulfide), nasal concha, rats. Localized congenital anomalies of the nasal cavity are rare in domestic animals and are often merely part of a more extensive craniofacial deformity (e.g., cyclops) or a component of generalized malformation (e.g., chondrodysplasia). Congenital anomalies involving the nasal cavity and sinuses, such as choanal atresia (lack of communication between the nasal cavity and pharynx), some types of chondrodysplasia, and osteopetrosis, are incompatible with life. Examples of nonfatal congenital anomalies include cystic nasal conchae, deviation of nasal septum, cleft upper lip (harelip, cheiloschisis), hypoplastic turbinates, and cleft palate (palatoschisis) (Fig. 7-1). Bronchoaspiration and aspiration pneumonia are common sequelae to cleft palate. Nasal and paranasal sinus cysts are slowly growing and expansive lesions that mimic neoplasia and cause severe cranial deformations in horses. These large cysts presumably originate congenitally from dentigerous tissue. As in other organs or systems, it is extremely difficult to determine the actual cause (genetic versus congenital) of anomalies based on pathologic evaluation. Congestion and Hyperemia: The nasal mucosa is well vascularized and is capable of rather dramatic variation in blood flow, whether passively as a result of interference with venous return (congestion) or actively because of vasodilation (hyperemia). Congestion of the mucosal vessels is a nonspecific lesion commonly found at necropsy and presumably associated with the circulatory failure preceding death (e.g., heart failure, bloat in ruminants in which the increased intraabdominal pressure causes increased intrathoracic pressure impeding the venous return from the head and neck). Hyperemia of the nasal mucosa is seen in early stages of inflammation, whether caused by irritations (e.g., ammonia, regurgitated feed), viral infections, secondary bacterial infections, allergy, or trauma. Hemorrhage: Epistaxis is the clinical term used to denote blood flow from the nose (nosebleed) regardless of whether the blood originates from the nasal mucosa or from deep in the lungs such as in horses with “exercise-induced pulmonary hemorrhage.” Unlike blood in the digestive tract, where the approximate anatomic location of the bleeding can be estimated by the color the blood imparts to fecal material, blood in the respiratory tract is always red. This fact is due to the rapid transport of blood out of the respiratory tract by the mucociliary blanket and during breathing. Hemorrhages into the nasal cavity can be the result of local trauma, originate from erosions of submucosal vessels by inflammation (e.g., guttural pouch mycosis), or be caused by neoplasms. Hemoptysis refers to the presence of blood in sputum or saliva (coughing or spitting blood) and is most commonly the result of pneumonia, lung abscesses, ulcerative bronchitis, pulmonary thromboembolisms, and pulmonary neoplasia. Ethmoidal (progressive) hematomas are important in older horses and are characterized clinically by chronic, progressive, often unilateral nasal bleeding. Grossly or endoscopically, an ethmoidal hematoma appears as a single, soft, tumorlike, pedunculated, expansive, dark red mass arising from the mucosa of the ethmoidal conchae (Fig. 9-12). Microscopic examination reveals a capsule lined by epithelium and hemorrhagic stromal tissue infiltrated with abundant macrophages, some of which are siderophages. Serous Rhinitis: Serous rhinitis is the mildest form of inflammation and is characterized by hyperemia and increased production of a clear fluid locally manufactured by serous glands present in the nasal submucosa. Serous rhinitis is of clinical interest only. It is caused by mild irritants or cold air, and it occurs during the early stages of viral infections, such as the common cold in humans, upper respiratory tract infections in animals, or in mild allergic reactions. Catarrhal Rhinitis: Catarrhal rhinitis is a slightly more severe process and has, in addition to serous secretions, a substantial increase in mucus production by increased activity of goblet cells and mucous glands. A mucous exudate is a thick, translucent, or slightly turbid viscous fluid, sometimes containing a few exfoliated cells, leukocytes, and cellular debris. In chronic cases, catarrhal rhinitis is characterized microscopically by notable hyperplasia of goblet cells. As the inflammation becomes more severe, the mucus is infiltrated with neutrophils giving the exudate a cloudy mucopurulent appearance. This exudate is referred to as mucopurulent. Purulent (Suppurative) Rhinitis: Purulent (suppurative) rhinitis is characterized by a neutrophilic exudate, which occurs when the nasal mucosa suffers a more severe injury that generally is accompanied by mucosal necrosis and secondary bacterial infection. Cytokines, leukotrienes, complement activation, and bacterial products cause exudation of leukocytes, especially neutrophils, which mix with nasal secretions, including mucus. Grossly, the exudate in suppurative rhinitis is thick and opaque, but it can vary from white to green to brown, depending on the types of bacteria and type of leukocytes (neutrophils or eosinophils) present in the exudate (Fig. 9-13 and Web Fig. 9-3). In severe cases, the nasal passages are completely blocked by the exudate. Microscopically, neutrophils can be seen in the submucosa and mucosa and form plaques of exudate on the mucosal surface. Neutrophils are commonly found marginated in vessels, in the lamina propria, and in between epithelial cells in their migration to the surface of the mucosa. Fibrinous Rhinitis: Fibrinous rhinitis is a reaction that occurs when nasal injury causes a severe increase in vascular permeability, resulting in abundant exudation of plasma fibrinogen, which coagulates into fibrin. Grossly, fibrin appears like a yellow, tan, or gray rubbery mat on nasal mucosa. Fibrin accumulates on the surface and forms a distinct film of exudate sometimes referred to as pseudomembrane (Fig. 9-14). If this fibrinous exudate can be removed, leaving an intact underlying mucosa, it is termed a croupous or pseudodiphtheritic rhinitis. Conversely, if the pseudomembrane is difficult to remove and leaves an ulcerated mucosa, it is referred to as diphtheritic or fibrinonecrotic rhinitis. The term diphtheritic was derived from human diphtheria, which causes a severe and destructive inflammatory process of the nasal, tonsillar, pharyngeal, and laryngeal mucosa. Microscopically, the lesions include a perivascular edema with fibrin, a few neutrophils infiltrating the mucosa, and superficial plaques of exudate consisting of fibrin strands mixed with leukocytes and cellular debris covering a necrotic and ulcerated epithelium. Fungal infections, such as aspergillosis, can cause a severe fibrinonecrotizing rhinitis. Fig. 9-14 Fibrinous rhinitis, midsagittal section of head, calf. Granulomatous Rhinitis: Granulomatous rhinitis is a reaction in the nasal mucosa and submucosa that is characterized by infiltration of numerous activated macrophages mixed with a few lymphocytes and plasma cells. In some cases, inflammation leads to the formation of polypoid nodules that in severe cases are large enough to cause obstruction of the nasal passages (Fig. 9-15). Granulomatous rhinitis is generally associated with chronic allergic inflammation or infection with specific organisms, such as those of the systemic mycoses (see the section on Lungs), tuberculosis, or rhinosporidiosis, and with foreign bodies. In some cases, the cause of granulomatous rhinitis cannot be determined. Fig. 9-15 Granulomatous rhinitis, midsagittal section of head, cow. Sinusitis: Sinusitis occurs sporadically in domestic animals and is frequently combined with rhinitis (rhinosinusitis), or it occurs as a sequela to penetrating or septic wounds of the nasal, frontal, maxillary, or palatine bones; improper dehorning in young cattle, which exposes the frontal sinus; or maxillary tooth infection in horses and dogs (maxillary sinus). Based on the type of exudate, sinusitis is classified as serous, catarrhal, fibrinous (rare), purulent, or granulomatous. Paranasal sinuses have poor drainage; therefore exudate tends to accumulate, causing mucocele (accumulation of mucus) or empyema (accumulation of pus) (Fig. 9-16). Chronic sinusitis may extend into the adjacent bone (osteomyelitis) or through the ethmoidal conchae into the meninges and brain (meningitis and encephalitis). Equine Viral Infections: Viruses, such as equine viral rhinopneumonitis virus, influenza virus, adenovirus, and rhinovirus, cause mild and generally transient respiratory infections in horses. The route of infection for these respiratory viruses is typically aerogenous. All these infections are indistinguishable clinically; signs consist mainly of malaise, fever, coughing, and nasal discharge varying from serous to purulent. Viral respiratory infections are common medical problems in adult horses. Equine viral rhinopneumonitis: Equine viral rhinopneumonitis (EVR) is caused by two ubiquitous equine herpesviruses (EHV-1 and EHV-4) and may be manifested as a mild respiratory disease in weanling foals and young racehorses, as a neurologic disease (myeloencephalopathy), or as abortion in mares. The portal of entry for the respiratory form is typically aerogenous, and the disease is generally transient; thus the primary viral-induced lesions in the nasal mucosa and lungs are rarely seen at necropsy unless complicated by secondary bacterial rhinitis, pharyngitis, or bronchopneumonia. Studies with polymerase chain reaction (PCR) techniques have demonstrated that, like other herpesvirus, EHV-1 and EHV-4 persist latently in the trigeminal ganglia for long periods of time. Reactivation because of stress or immunosuppression and subsequent shedding of the virus are the typical source of infection for susceptible animals on the farm. Equine influenza: Equine influenza is a common, highly contagious, and self-limiting upper respiratory infection of horses caused by aerogenous exposure to type A strains of influenza virus (H7N7 [A/equi-1] and H3N8 [A/equi-2]). Equine influenza has high morbidity (outbreaks) but low mortality, and it is clinically characterized by fever, conjunctivitis, and serous nasal discharge. It occurs mainly in 2- to 3-year-old horses at the racetrack. As with human influenza, equine influenza is usually a mild disease, but occasionally it can cause severe bronchointerstitial pneumonia with pulmonary edema. In some horses, impaired defense mechanisms caused by the viral infection are complicated by a secondary bacterial bronchopneumonia caused by opportunistic organisms (Streptococcus zooepidemicus, Staphylococcus aureus, or Bacteroides sp.) found in the normal flora of the upper respiratory tract. Uncomplicated cases of equine influenza are rarely seen in the postmortem room. Other equine respiratory viruses: Equine rhinovirus, adenovirus, and parainfluenza virus produce mild and transient upper respiratory infections (nasopharynx and trachea) in horses, unless complicated by secondary pathogens. In addition to reduced athletic performance, infected horses may have a temporary suppression of cell-mediated immunity leading to opportunistic infections such as Pneumocystis carinii pneumonia. Fatal adenoviral infections with severe pneumonia or enteritis occur commonly in immunocompromised horses, particularly in Arabian foals with inherited combined immunodeficiency disease. Equine Bacterial Infections: Strangles, Glanders, and Melioidosis: Strangles, glanders, and melioidosis of horses are all systemic bacterial diseases that cause purulent rhinitis and suppuration in various organs. These diseases are grouped as upper respiratory diseases because nasal discharge is often the most notable clinical sign. Strangles: Strangles is an infectious and highly contagious disease of Equidae that is caused by Streptococcus equi ssp. equi (Streptococcus equi). It is characterized by suppurative rhinitis and lymphadenitis (mandibular and retropharyngeal lymph nodes) with occasional hematogenous dissemination to internal organs. Unlike Streptococcus equi ssp. zooepidemicus (Streptococcus zooepidemicus) and Streptococcus dysgalactiae ssp. equisimilis (Streptococcus equisimilis), Streptococcus equi is not part of the normal nasal flora. Infection occurs when susceptible horses come into contact with feed, exudate, or air droplets containing the bacterium. After penetrating through the nasopharyngeal mucosa, Streptococcus equi drains to the regional lymph nodes—mandibular and retropharyngeal lymph nodes—via lymphatic vessels. The gross lesions in horses with strangles (mucopurulent rhinitis) correlate with clinical findings and consist of copious amounts of mucopurulent exudate in the nasal passages with notable hyperemia of nasal mucosa. Affected lymph nodes are enlarged and may contain abscesses filled with thick purulent exudate (purulent lymphadenitis). The term bastard strangles is used in cases in which hematogenous dissemination of Streptococcus equi results in metastatic abscesses in such organs as the lungs, liver, spleen, kidneys, or brain or in the joints. This form of strangles is often fatal.
Respiratory System, Mediastinum, and Pleurae
Structure and Function
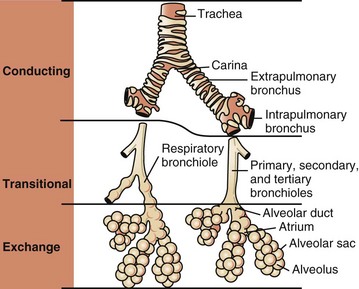
Conducting, transitional, and exchange components of the respiratory system. The transitional zone (bronchioles) is not as equally well developed in all species. (From Banks WJ: Applied veterinary histology, ed 3, St Louis, 1993, Mosby.)
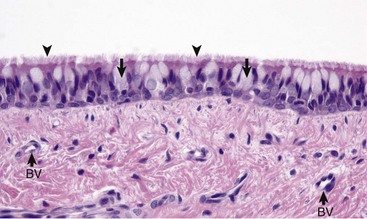
Mucosa consists of ciliated and nonciliated secretory cells. Goblet cells have a pale staining cytoplasm (arrows). The proportion of ciliated to nonciliated cells varies depending on the level of airways. Ciliated cells (arrowheads) are more abundant in proximal airways, whereas secretory cells are proportionally more numerous in distal portions of the conducting and transitional systems. The submucosa of the conducting system (nasal to bronchi) has abundant blood vessels (BV). (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
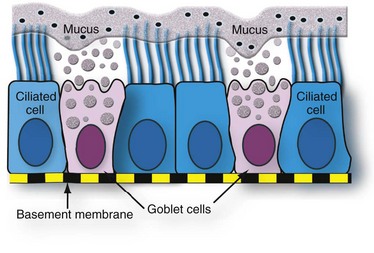
Both ciliated and goblet cells rest on the basement membrane. Mucus produced and released by goblet cells forms a carpet on which inhaled particles (dots) are trapped and subsequently expelled into the pharynx by the mucociliary apparatus. (Courtesy Dr. A. López, Atlantic Veterinary College.)
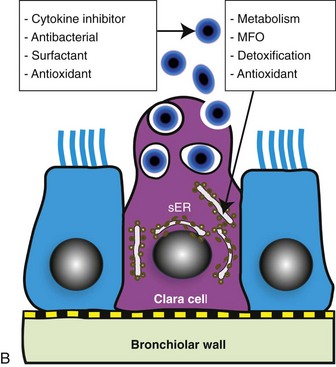
A, Bronchiole showing a thin wall composed of a basement membrane, smooth muscle, and connective tissue. On the luminal surface of the bronchiole note dome-shaped Clara cells (arrows) protruding into the lumen. H&E stain. B, Schematic representation of a Clara cell showing abundant smooth endoplasmic reticulum (SER) and cytoplasmic granules, which are extruded into the bronchiolar lumen. MFO, Mixed function oxidases. (Courtesy Dr. A. López, Atlantic Veterinary College.)
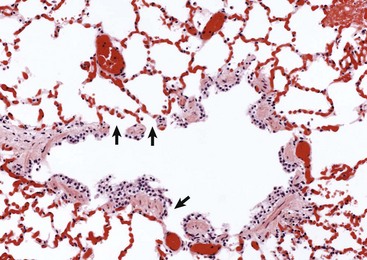
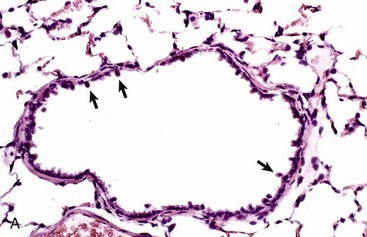
The wall of the bronchiole is covered by ciliated epithelium, which is supported by smooth muscle and connective tissue. Terminally, the wall becomes interrupted, forming lateral communications between the bronchiolar lumen and alveoli (arrows). (Courtesy Dr. A. López, Atlantic Veterinary College.)
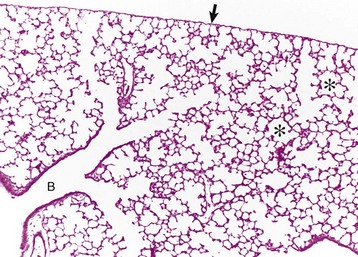
Lungs were fixed by intratracheal perfusion of fixative to retain normal distention of airways. Note the dichotomous branching of the bronchioles and the thin visceral pleura (arrow) covering the surface of the lungs (B) that terminate as alveoli (asterisks). H&E stain. (Courtesy Dr. J. Martinez-Burnes, Atlantic Veterinary College.)
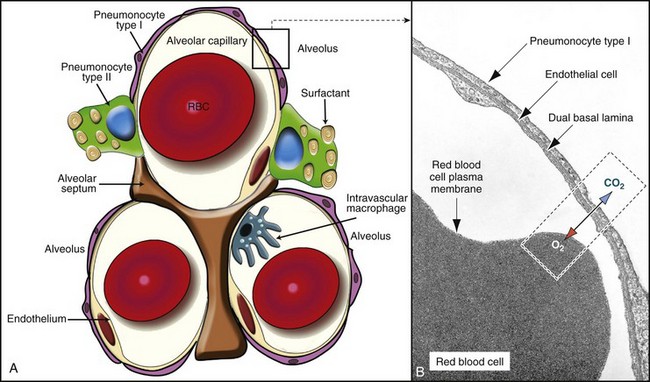
A, In this schematic diagram, note the thin membrane (blood-air barrier) separating the blood compartment from the alveoli. Type I alveolar cells (membranous pneumonocytes) are remarkably thin and cover most of the alveolar wall. Note the endothelial cells lining the alveolar capillary. Alveolar interstitium supports the alveolar epithelium on one side and the endothelium on the other side of the blood-air barrier. Type II (granular) pneumonocytes appear as large cuboidal cells with lamellar bodies (surfactant) in the cytoplasm. A pulmonary intravascular macrophage, a component of the monocyte-macrophage system, is depicted on the wall of an alveolar capillary. A red blood cell (RBC) is present inside the lumen of the alveolar capillary. B, Alveolar wall. The blood-air barrier consists of cytoplasmic extensions of (1) type I alveolar cells (membranous pneumonocytes); (2) a dual basal lamina synthesized by type I alveolar cells; (3) cytoplasmic extensions of endothelial cells. TEM. Uranyl acetate and lead citrate stain. (A courtesy Dr. A. López, Atlantic Veterinary College. B from Kierszenbaum AL: Histology and cell biology, St Louis, 2002, Mosby.)
Category
Agents
Microbes
Viruses, Chlamydophila, bacteria, fungi, protozoa
Plant dust
Grain, flour, cotton, wood
Animal products
Dander, feathers, mites, insect chitin
Toxic gases
Ammonia (NH3), hydrogen sulfide (H2S), nitrogen dioxide (NO2), sulfur dioxide (SO2), chlorine
Chemicals
Organic and inorganic solvents, herbicides, asbestos, nickel, lead
Portals of Entry into the Respiratory System
Route
Agents
Aerogenous (inhalation)
Virus, bacteria, Chlamydophila, fungi, toxic gases, and pneumotoxicants
Hematogenous (blood)
Virus, bacteria, fungi, parasites, toxins, and pneumotoxicants
Direct extension
Penetrating wounds, migrating awns, bites, and ruptured esophagus or perforated diaphragm (hardware)
Defense Mechanisms of the Respiratory System
Regions of the Respiratory System
Defense Mechanisms
Conducting system (nose, trachea, and bronchi)
Mucociliary clearance, antibodies, lysozyme, mucus
Transitional system (bronchioles)
Clara cells, antioxidants, lysozyme, antibodies
Exchange system (alveoli)
Alveolar macrophages (inhaled pathogens), intravascular macrophages (circulating pathogens), opsonizing antibodies, surfactant, antioxidants
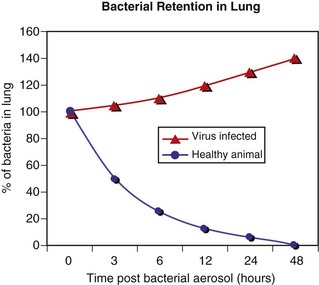
When large numbers of bacteria are inhaled, the normal defense mechanisms promptly eliminate these microorganisms from the lungs (blue line). However, when the defense mechanisms are impaired by a viral infection, lung edema, stress, and so forth, the inhaled bacteria are not eliminated but colonize and multiply in the lung (red line). (Courtesy Dr. A. López, Atlantic Veterinary College.)
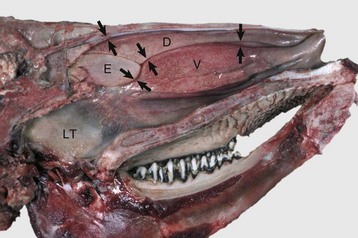
These meatuses (spaces between arrows) are narrow and the air turbulence produced in them by the coiled arrangement of the conchae causes suspended particles to impact on the mucus covering the surface of the nasal mucosa. These particles are then moved caudally by the mucociliary apparatus to the pharynx and finally swallowed. Note the abundant lymphoid tissue (LT) in the nasopharynx. (Courtesy Dr. R.G. Thomson, Ontario Veterinary College.)
Defense Mechanisms of the Conducting System (Nose, Trachea, and Bronchi)
Defense Mechanisms of the Exchange System (Alveoli)
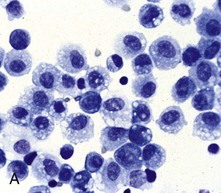
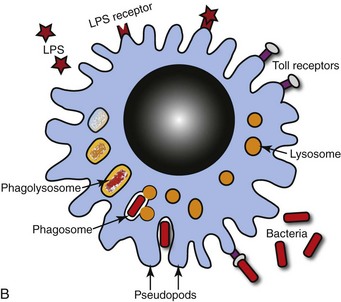
A, Bronchoalveolar lavage, healthy pig. Alveolar macrophages characterized by abundant and vacuolated cytoplasm are the predominant cell in lavages from healthy lungs. Mayer’s hematoxylin counter stain. B, Schematic representation of a pulmonary alveolar macrophage. Note receptors in cell membrane, attachment of bacteria to cell receptor, bacteria being engulfed by cytoplasmic projections (pseudopods), formation of cytoplasmic phagosomes, and fusion of lysosomes with phagosome (phagolysosomes) which finally kill the ingested bacteria. (A courtesy Dr. L.A. Rijana-Ludbit, Tübingen; B courtesy of Dr. A. López, Atlantic Veterinary College.)
Cell/Secretory Product
Action
Alveolar macrophage
Phagocytosis, main line of defense against inhaled particles and microbial pathogens in the alveoli
Intravascular macrophage
Phagocytosis, removal of particles, endotoxin, and microbial pathogens in the circulation
Ciliated cells
Expel mucus and inhaled particles and microbial pathogens by ciliary action
Clara cells
Detoxification of xenobiotics (mixed function oxidases) and protective secretions against oxidative stress and inflammation; production of surfactant
Mucus
Physical barrier; traps inhaled particles and microbial pathogens and neutralizes soluble gases
Surfactant
Protects alveolar walls and enhances phagocytosis
Lysozyme
Antimicrobial enzyme
Transferrin and lactoferrin
Inhibition and suppression of bacterial growth
α1-Antitrypsin
Protects against the noxious effects of proteolytic enzymes released by phagocytic cells; also inhibits inflammation
Interferon
Antiviral agent and modulator of the immune and inflammatory responses
Interleukins
Chemotaxis, upregulation of adhesion molecules
Antibodies
Prevent microbe attachment to cell membranes, opsonization
Complement
Chemotaxis; enhances phagocytosis
Antioxidants*
Prevent injury caused by superoxide anion, hydrogen peroxide, and free radicals (ROS) generated during phagocytosis, inflammation, or by inhalation of oxidant gases (ozone, nitrogen dioxide [NO2], sulfur dioxide [SO2])
Defense Mechanisms Against Blood-Borne Pathogens (Intravascular Space)
Impairment of Defense Mechanisms in the Respiratory System
Viral Infections
Structure and Function

A, Normal bronchial mucosa, bronchus, rat. The mucous layer was removed before fixation to expose the external surface of the epithelium. Mucosa consists of ciliated cells and nonciliated secretory cells. Ciliated cells have numerous slender cilia (arrows). Nonciliated secretory cells have a dome-shaped surface with abundant microvilli (arrowheads). The proportion of ciliated to nonciliated cells varies depending on the level of airways. Ciliated cells are more abundant in proximal airways, whereas secretory cells are more numerous in distal portions of the conducting and transitional systems. Scanning electron micrograph. Carbon-sputter coating method. B, Normal ciliated epithelium, trachea, cow. This trachea was specially fixed to preserve the mucous layer, which consists of an internal, clear, hypophase-fluid layer (not visible here) surrounding microvilli and kinocilia and an external mucous epiphase at the level of the tips of the kinocilia (cut in both transverse and longitudinal section here). TEM. Uranyl acetate and lead citrate stain. (A courtesy Dr. A. López, Atlantic Veterinary College. B from Sims DE, Westfall JA, Kiorpes AL, Horne MM: Biotech Histochem 66:173-180, 1991.)
Examination of the Respiratory Tract
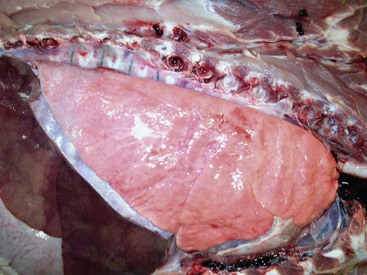
The lung parenchyma appears homogeneously pink. The pale pink appearance of these normal lungs is due to exsanguination. The appearance of normal unexsanguinated lungs is bright pink to red. (Courtesy Dr. A. López, Atlantic Veterinary College.)
Bronchoalveolar Lavage and Tracheal Aspirates
Diseases of the Respiratory System
Pattern of Injury and Host Response
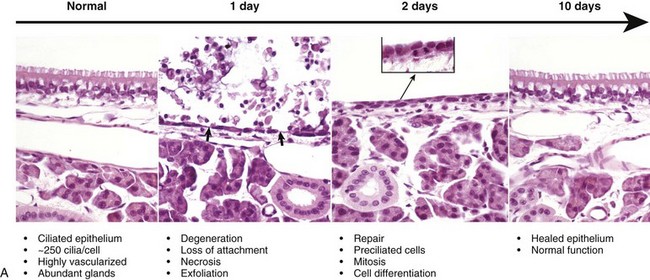

A, Normal ciliated epithelium composed of tall columnar cells with numerous cilia. Day 1. Note detachment and exfoliation of ciliated cells, leaving a denuded basement membrane (arrows). This same type of lesion is seen in viral or mechanical injury to the mucosa of the conducting system. Two days after exposure, the basement membrane is lined by rapidly dividing preciliated cells, some of which exhibit mitotic activity (inset). Ten days after injury, the nasal epithelium is completely repaired. H&E stain. B, Schematic representation of the events of injury and repair in the respiratory mucosa of the conducting system. Blue cell = ciliated mucosal epithelial cell; pink cell = goblet cell; red cell = neutrophil. (A from López A, Prior M, Yong S et al: Am J Vet Res 49:1107-1111, 1988; B courtesy of Dr. A. López, Atlantic Veterinary College.)
Anomalies of the Nasal Cavity
Circulatory Disturbances of the Nasal Cavity
Inflammation of the Nasal Cavity
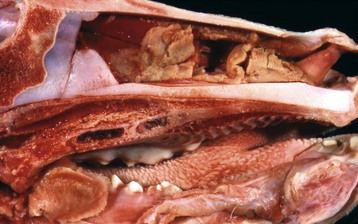
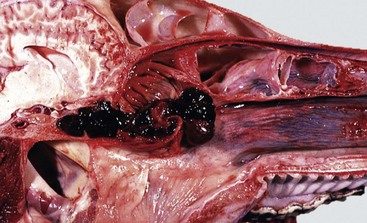
Infectious bovine rhinotracheitis (IBR; bovine herpesvirus 1). The nasal septum has been removed to expose nasal conchae. The nasal mucosa is covered by diphtheritic yellow membranes consisting of fibrinonecrotic exudate. Removal of these fibrinous membranes reveals focal ulcers in the underlying mucosa. (Courtesy Dr. Scott McBurney, Atlantic Veterinary College.)
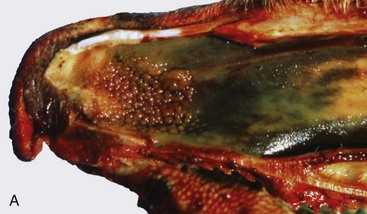
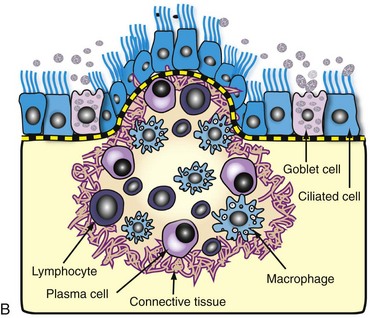
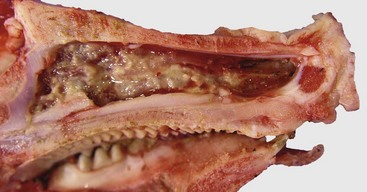
A, Note multiple and often confluent granulomas arising from the nasal mucosa. B, Schematic representation of a nasal granuloma showing the outer wall of the granuloma composed of connective tissue enclosing the center, which has been infiltrated with lymphocytes, plasma cells, and macrophages. (A courtesy Ontario Veterinary College. B courtesy of Dr. A. López, Atlantic Veterinary College.)
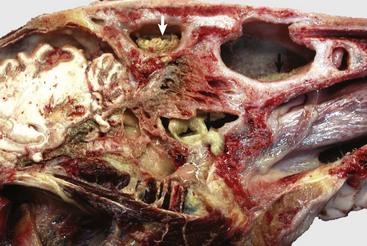
Specific Diseases of the Nasal Cavity and Sinuses
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree