CHAPTER 12 Reproductive System
FEMALE REPRODUCTIVE SYSTEM: MAMMARY GLANDS, OVARIES, UTERUS, AND VAGINA
Mammary Glands
Mammary gland lesions are common in female dogs and cats. Mammary gland enlargement may be related to a wide variety of disease processes, including cysts, inflammation, hyperplasia, and benign or malignant neoplasia. Important information in the investigation of mammary gland disease includes history, breed, and age; whether the gland was intact or older when the dog or cat was neutered; date of last estrus, pregnancy, or hormone therapy; size, number, and consistency of lesion(s); attachment to underlying tissue; rate of growth; presence of ulceration; and evidence of metastasis (Baker and Lumsden, 1999). Ancillary diagnostic tests used to evaluate mammary lesions include a thorough evaluation of health status involving a complete physical examination, complete blood count, serum biochemical profile, urinalysis and/or coagulation profile, imaging, cytology, and histopathology.
Normal Anatomy and Histology
Mammary glands are compound tubuloalveolar glands that are believed to be extensively modified sweat glands (Banks, 1986). In dogs and cats, five pairs of mammary glands are arranged as bilaterally symmetrical rows extending from the ventral thorax to the inguinal region. During pregnancy and lactation, the mammary glands undergo marked hypertrophy and hyperplasia to produce immunoglobulin-containing colostrum followed by milk.
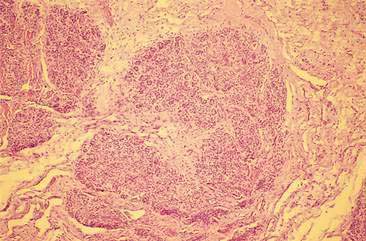
Histologically, mammary glands are composed of a secretory component consisting of alveolar secretory epithelial cells and the initial portion of the intralobular ducts (Banks, 1986) (Figs. 12-1 to 12-3). The secretory portion of the glands is drained by the ductular system, composed of nonsecretory columnar and cuboidal epithelium. Reticular connective tissue supports the alveoli and smaller ducts. Bundles of smooth muscle and elastic fibers surround the large ducts. Myoepithelial cells can be found between the alveolar epithelial cells and the underlying basement membrane. The normal histology of mammary gland from immature female dogs and sequential microscopic changes that occur during different stages of the estrous cycle are reviewed elsewhere (Rehm et al., 2007).
Normal Cytology
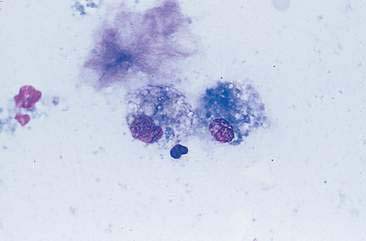
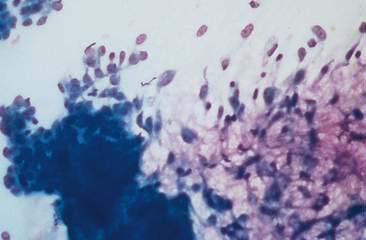
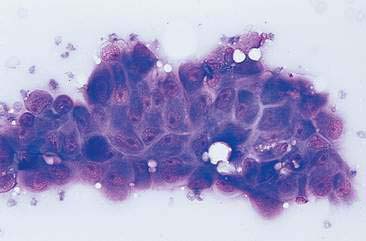
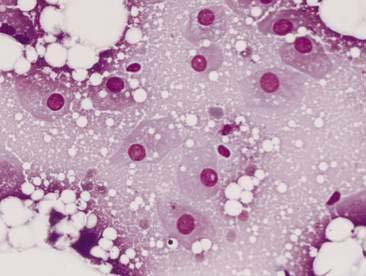
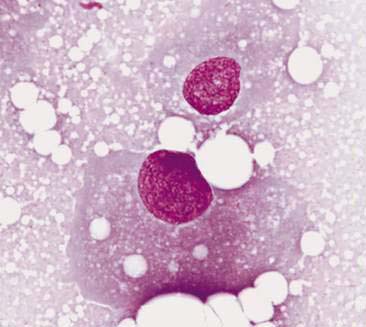
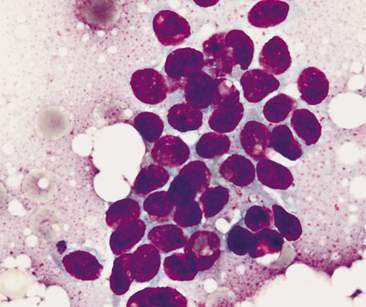
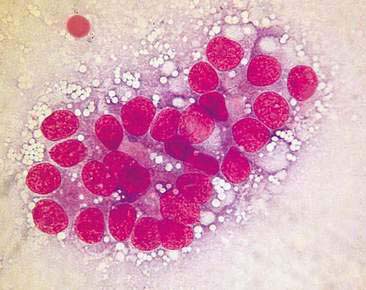
Normal mammary secretions are characterized cytologically by low numbers of sloughed secretory epithelial cells known as foam cells as well as macrophages and occasional neutrophils on an eosinophilic to basophilic proteinaceous background. Foam cells are large, individualized cells characterized by round to oval, eccentrically located nuclei and abundant amounts of vacuolated cytoplasm (Allison and Maddux, 2008). These cells may also contain amorphous, basophilic secretory material (Fig. 12-4). Foam cells resemble and can be difficult to distinguish from reactive macrophages. FNA cytology of normal mammary tissue usually reveals small amounts of blood with no to low numbers of nucleated cells and moderate to large amounts of basophilic, proteinaceous material, clear lipid droplets, and adipocytes (Allen et al., 1986). Small sheets and clusters of mammary secretory epithelial cells that are uniform in size and shape may be seen occasionally in aspirates of normal mammary tissue. Secretory epithelial cells exhibit round, dark nuclei and moderate amounts of basophilic cytoplasm. Acinar formations may be noted. Ductular epithelial cells are characterized by oval, basal nuclei with scant amounts of cytoplasm. Myoepithelial cells may be seen as darkly staining, oval free nuclei or as spindle-shaped cells (Allison and Maddux, 2008).
Mammary Cysts
Mammary cysts or fibrocystic disease (FCD), also known as blue dome cyst or polycystic mastopathy, is a form of mammary dysplasia in which dilated ducts expand to form cavitary lesions (Brodey et al., 1983). FCD generally occurs in middle-aged to older animals, although the disease has been reported in dogs of 1 year of age. Formation of FCD may have a hormonal component as administration of medroxyprogesterone has been associated with development of FCD in dogs. In dogs, rapid growth during estrus and regression during metestrus has been noted. The rapid growth of cysts during estrus may be associated with rupture of the cysts. Ovariohysterectomy should be considered when mammary cysts grow and regress in association with the estrous cycle, particularly if multiple glands are involved. FCD is considered a benign lesion in dogs; however, the disease has been associated with development of mammary gland carcinoma.
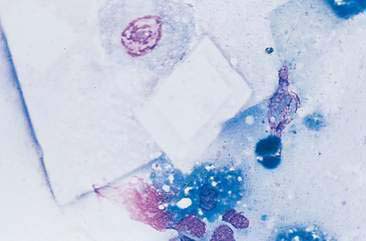
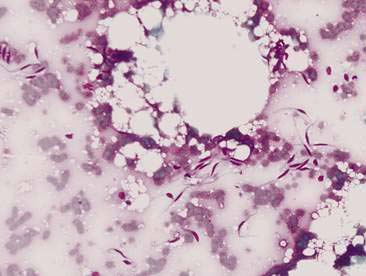
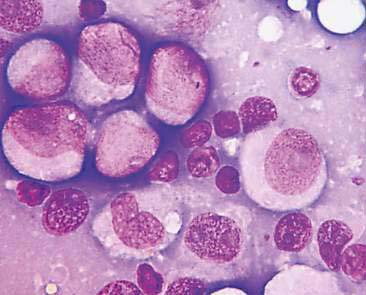
Mammary cysts may present as a well-circumscribed, single cystic nodule or as a flat, rubbery, multinodular mass. The nodule(s) exhibit slow, expansile growth and the overlying skin may develop a blue color, hence the term blue dome cyst (Brodey et al., 1983). Mammary cysts may be classified as simple cysts characterized by a single layer of flattened lining epithelium or papillary cysts containing papillary outgrowths of the lining epithelial cells. Aspiration of mammary cysts typically yields a green-brown or blood-tinged fluid containing low numbers of foam cells and pigment-laden macrophages (Allison and Maddux, 2008). Neutrophils may be increased if inflammation is also present. Cholesterol crystals, which appear as large, rectangular crystalline structures often with a notched corner, may be present as a result of breakdown of cellular membranes within the cyst (Fig. 12-5). Epithelial cells derived from the cystic lining may be noted, particularly if the cyst has a papillary component. These cells tend to occur in dense sheets and clusters and may display some mild variation in nuclear size and shape. Mammary cysts may coexist with benign and/or malignant mammary tumors (Brodey et al., 1983). Therefore, aspiration or biopsy of solid areas of a mass associated with a cyst or other mammary masses should be performed to rule out the presence of concurrent mammary neoplasia.
Mammary Gland Hyperplasia
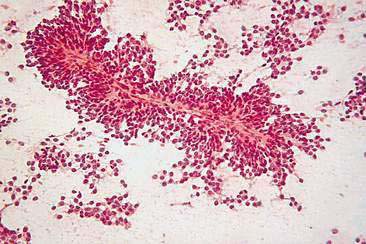
Hyperplastic and dysplastic lesions of mammary glands include unilobular and multilobular hyperplasia, adenosis, and epitheliosis (Misdorp et al., 1999). These lesions occur in dogs and less commonly in cats (Yager et al., 1993). Mammary hyperplasia is characterized by proliferations of secretory or ductular epithelium or myoepithelial cells resembling the physiologic hyperplasia of pregnancy with some mild histologic atypia. Cytologically, these lesions may be difficult to distinguish from each other and from benign neoplasms such as adenomas or papillomas. Moderate to large numbers of epithelial cells arranged in sheets and clusters can be aspirated from hyperplastic mammary tissue. These cells, which are similar in appearance to normal mammary epithelial cells, display round nuclei with fine to lightly stippled chromatin of uniform size and shape and scant to moderate amounts of basophilic cytoplasm. Foam cells and macrophages may also be noted.
In cats, a form of mammary hyperplasia occurs that has been variously identified as fibroepithelial hyperplasia, feline mammary hypertrophy, mammary fibroadenomatous hyperplasia, or feline mammary hypertrophy/fibroadenomatous complex. Feline mammary fibroepithelial hyperplasia (MFH) is a clinically benign, fairly common condition affecting estrous-cycling or pregnant female cats usually less than 2 years of age (Mesher, 1997). MFH has also been reported in older intact and neutered cats of either gender receiving progesterone-containing compounds, such as megestrol acetate (Hayden et al., 1989) or depot medroxyprogesterone acetate (Loretti et al., 2005). MFH is considered a form of mammary dysplasia characterized by a rapid, abnormal growth of one or more mammary glands. In contrast to a neoplastic process, paired glands often exhibit a similar degree of enlargement (Lana et al., 2007). MFH is notable for a marked intralobular ductular proliferation identical to the ductular proliferation seen during the progesterone-influenced early stages of pregnancy (Misdorp et al., 1999). This typical histologic appearance, the occurrence in cycling females or cats administered progesterone, and the identification of progesterone receptors in MFH lesions from female and male cats have led to the belief that development of MFH involves endogenous or exogenous progesterone. MFH usually regresses over time without treatment, although secondary infections may require appropriate antibiotic therapy. Ovariohysterectomy, performed via a flank incision if the glands are greatly enlarged, will often result in regression of lesions and will prevent future recurrences (Lana et al., 2007). However, some cats do not respond to withdrawal of progestogens or ovariectomy and can be treated successfully with the progesterone receptor blocker aglépristone (Görlinger et al., 2002).
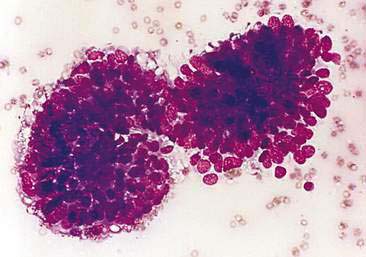
The cytologic appearance of MFH (Fig. 12-6) has been reported (Mesher, 1997). Aspirated material of a histologically confirmed MFH lesion consisted of a very uniform population of cuboidal epithelial cells arranged in thick clusters. The cuboidal epithelial cells were characterized by dense, round nuclei with small nucleoli and scant amounts of basophilic cytoplasm. A mesenchymal population of spindle-shaped cells with narrow oval nuclei, one to two nucleoli, and tapering cytoplasm was also present. The mesenchymal cells displayed moderate variation in nuclear size (anisokaryosis) and cellular size (anisocytosis). Moderate amounts of pink extracellular matrix were associated with the mesenchymal cells. These cytologic findings correlated with the histologic findings of hyperplastic ductular epithelium (cuboidal epithelial population) and proliferation of edematous stroma (mesenchymal cells with extracellular matrix). The presence of abundant stromal elements helps to differentiate MFH from mammary neoplasia, which generally contains scant stromal material. Cytologic recognition of the characteristic cell types from mammary masses in a cat with appropriate signalment and clinical history can be considered highly suggestive of MFH, thus eliminating the need for mammary gland excision and allowing for appropriate medical and/or surgical management.
Mammary Gland Inflammation/Infection
Inflammation of the mammary glands is referred to as mastitis and may present as a focal lesion or may involve one or more glands. Mastitis may infrequently occur from hematogenous spread of organisms, nonlactation-associated trauma, fight wounds, or infected neoplasms. Dirofilaria repens infection of the mammary gland in the bitch (Manuali et al., 2005) and mastitis due to Toxoplasma gondii in a cat (Park et al., 2007) have been recently reported. Mastitis is most often associated with postparturient lactation. It can also occur during pseudopregnancy, as well after early weaning of puppies. It is thought to result from entry of infectious organisms through the teat orifice or damaged overlying skin (Gruffydd-Jones, 1980). Neonatal morbidity or mortality may be the first indication of mastitis. Clinical signs associated with mastitis include swollen, painful glands that result in discomfort while nursing. The glands may become abscessed or gangrenous with necrosis of overlying skin. The bitch or queen may also present with clinical signs of systemic illness such as anorexia, fever, vomiting, or diarrhea. A complete blood count may reveal an inflammatory leukogram characterized by either an increase in segmented and nonsegmented (band) neutrophils or a degenerative left shift with a predominance of immature neutrophils, especially if gangrenous mastitis is present (Ververidis et al., 2007).
Cytologic examination of secretions from inflamed and/or infected mammary glands is usually diagnostic; however, FNA may be needed for focal lesions. Large numbers of neutrophils are present, which may exhibit degenerative changes of karyolysis and karyorrhexis. Reactive macrophages, small lymphocytes, and plasma cells may also be seen, particularly with more chronic lesions. Infectious organisms may be visualized within neutrophils and, less commonly, macrophages, indicating a septic process. Various bacteria have been incriminated as etiologic agents of disease such as Staphylococcus spp., Streptococcus spp., and E. coli. Other types of bacteria and fungi can be isolated (Allison and Maddux, 2008). Staphylococcus intermedius is the most common cause of clinical and subclinical mastitis in the dog (Schafer-Somi et al., 2003). Culture and sensitivity of milk, inflamed mammary secretions, or aspirated material are warranted to determine appropriate antibiotic therapy.
Some noninfectious inflammatory conditions of mammary glands have been described. Focal mastitic lesions may leave residual fibrotic nodules consisting of epithelial cell metaplasia, pigment-laden macrophages, nondegenerate neutrophils, small lymphocytes, and plasma cells (Allison and Maddux, 2008). Unlike mammary gland tumors, fibrotic nodules tend to occur in young dogs, do not increase in size, and are usually associated with a previous history of mastitis (Brodey et al., 1983).
Neoplasia
Canine Mammary Gland Tumors
Following skin tumors, mammary neoplasms are the second most common tumor in dogs and the most commonly seen tumor in bitches (Misdorp, 2002). Mammary gland tumors (MGT) rarely occur in male dogs with a reported annual incidence of 4 in 100,000 while the annual incidence is 207 in 100,000 in female dogs (Saba et al., 2007; Lana et al., 2007). Many of the MGT reported in male dogs have been associated with small tumor sizes, benign or well-differentiated malignant epithelial tumors, nondefinitive evidence of metastatic disease at diagnosis, and intense estrogen-receptor positivity. The median age for development of canine mammary gland tumors is 10 to 11 years of age, with rare occurrence in bitches younger than 4 years old. Breed tendencies for MGT have been reported with a predisposition in several spaniels breeds, the Poodle, Dachshund, and other breeds (Sorenmo, 2003) and with a greater prevalence of malignant tumors in large breeds than in small breeds (Itoh et al., 2005). A heritable, familial tendency for development of mammary neoplasms in Beagles has been suggested (Benjamin et al., 1999). Development of MGT appears to have a hormonal component as evidenced by the sparing effect of ovariohysterectomy in first estrus cycles and by the increased length of survival time in dogs spayed less than 2 years before mammary carcinoma surgery when compared with dogs spayed longer than 2 years before tumor surgery or intact dogs (Sorenmo et al., 2000). Estrogen and progesterone receptors have been identified in normal, hyperplastic/dysplastic mammary tissue and a majority of mammary neoplasms (Lana et al., 2007; Millanta et al., 2005; de las Mulas et al., 2005). Interestingly, hormone receptor expression, which is a characteristic feature of mature mammary epithelial cells, tends to be decreased or absent in poorly differentiated tumors and metastatic lesions. It is well known that progesterone or synthetic progestins administration increases the incidence of MGT in dogs (Misdorp, 1991). Mechanisms involved in the progesterone-induced mammary gland tumors include an upregulation of growth hormone production by mammary epithelial cells (van Garderen and Schalken, 2002) and a rise in blood levels of insulin-like growth factor I (IGF-I) and IGF-II (Lana et al., 2007). Growth hormone and IGF may increase proliferation of susceptible or transformed mammary epithelial cells, resulting in neoplasia. Other risk factors to develop MGT are obesity at 1 year of age and low-fat/low-protein diet (Sorenmo, 2003). Other molecule targets have been investigated to elucidate prognosis or the pathways of tumorigenesis such as cyclooxygenase-2 (Millanta et al., 2006a), heat-shock proteins (Romanucci et al., 2006), VEGF (Millanta et al., 2006b), p53, BRCA1, c-erbB-2, antiapoptotic and proapoptotic proteins (Lana et al., 2007), beta-catenin, E-cadherin and Adenomatous Polyposis Coli (APC) (Restucci et al., 2007) and connexin (Torres et al., 2005), as well as several proliferation markers such as proliferating cell nuclear antigen (PCNA) and Ki-67 (Lana et al., 2007). Immunocytochemical Ki-67 marker seems to be useful to identify malignant canine tumors and patient poor outcome (Zuccari et al., 2004).
Mammary tumors can present as single, firm, wellcircumscribed masses to multiple, infiltrative nodules involving one or more glands. In animals with benign mammary tumors, the tumor is small, well circumscribed, and firm on palpation. Clinical findings associated with malignant neoplasms include a tumor diameter greater than 5 cm, recent rapid growth, ill-defined boundaries, infiltration of surrounding tissue, erythema, ulceration, inflammation, and edema. However, most benign and malignant canine mammary tumors exhibit none of these signs with the exception of dogs with advanced metastatic disease or inflammatory mammary carcinomas that typically have systemic signs of illness when they are diagnosed (Lana et al., 2007).
The majority of mammary neoplasms occur primarily in the caudal glands, presumably because of the larger amount of glandular tissue present (Sorenmo, 2003). Multiple mammary neoplasms are common, with 50% to 60% of dogs presenting with more than one mammary tumor. Multiple MGT in a dog are often not of the same histologic type and may exhibit differing biologic behaviors (Benjamin et al., 1999). Thus, a thorough search for additional tumors should be undertaken if a mammary mass is found, and separate cytologic and/or histologic analyses should be performed on each mammary tumor.
The ultimate goal of clinical, cytologic, and histologic evaluation of mammary gland neoplasms is to accurately predict the biologic behavior of the tumor. The World Health Organization International Histological Classification of Mammary Tumors of the dog and the cat combines histiogenic and descriptive morphologic classification, incorporating histologic prognostic features that have been associated with increasing malignancy (Misdorp et al., 1999). Most mammary gland tumors are of epithelial origin. Some tumors are composed of both epithelial and myoepithelial tissue, with areas of cartilage and bone, and a few tumors are of purely mesenchymal origin. About 50% of canine MGT have been classified as malignant based on histologic appearance (Brodey et al, 1983). While some classifications of mammary gland tumors, such as carcinosarcomas or sarcomas, have a consistently poor prognosis, histologic evidence of malignancy does not always imply a malignant course (Lana et al., 2007). In fact, only 50% of histologically diagnosed mammary carcinomas result in tumor-associated deaths (Brodey et al., 1983). Morphologic criteria of malignancy, such as cellular pleomorphism, mitotic activity, and individual grades of anaplasia, are not sufficient criteria for diagnosis of carcinomas. Instead, infiltration into skin and soft tissues and invasion of tumor cells into blood or lymphatic vessels have been identified as best histologic evidence of malignancy in mammary tumors (Misdorp, 2002). When stromal invasion is present, 80% of affected dogs will be dead within 2 years while, in the absence of stromal invasion, 80% of affected dogs will be alive after 2 years (Yager et al., 1993). Using stromal invasion as the primary criteria for malignancy, a lifespan study of over a thousand beagles was reported that correlated the various histologic classifications of epithelial mammary tumors with biologic behavior (Benjamin et al., 1999). Specifically, the study showed that ductular carcinomas accounted for 65.8% of all fatalities due to mammary neoplasia, even though these tumors composed only 18.7% of all mammary carcinomas. Of the malignant tumors, squamous cell carcinomas exhibited the lowest metastatic rate (20%), with carcinosarcomas exhibiting the highest rate of metastasis (100%). Ductular carcinomas metastasized more frequently than adenocarcinomas, 45% versus 35%, respectively.
Accurate and diagnostic exfoliative cytology of mammary tumors is associated with difficulties. Mesenchymal tumors or tumors with a fibrous or scirrhous component may not exfoliate well, leading to a poorly cellular sample inadequate for diagnosis. Tissue imprints or smears of tissue scrapings taken from biopsy samples may improve cytologic diagnosis in these cases; however, imprints generally do not yield as good a sample for evaluation as aspirates (Baker and Lumsden, 1999). Also, mammary hyperplasia, dysplasia, benign tumors, and well-differentiated carcinomas tend to form a continuum of morphologic appearance, making cytologic differentiation of these lesions difficult (Benjamin et al., 1999). Lastly, the presence of stromal invasion, one of the most important criteria for determining the malignant potential of a mammary neoplasm, cannot be assessed by the cytologist. All of these factors can result in either false-positive or false-negative diagnosis of malignant mammary tumors using aspiration cytology. A few studies have examined the accuracy of cytology for detecting mammary malignancies as compared to histologic findings. Allen et al (1986) reported cytologic sensitivities for detecting malignancies of 25% and 17% and specificities of 62% and 49% for the two cytopathologists involved in the study. Positive (PPV) and negative (NPV) predictive values were generally similar between the two pathologists, with PPVs of 90% and 100% and NPVs of 75% and 59% (Allen et al., 1986). The diagnostic accuracy was reported as 79% and 66%. In another study, the sensitivity for cytologic detection of mammary malignancies was found to be 65% with a specificity of 94% (Hellman and Lindgren, 1989). The PPV was reported as 93% with an NPV of 67% and diagnostic accuracy of 79%. In a recent study, cytologic and histologic diagnostic agreement was 67.5%. However, when suspicious and insufficient/inadequate samples were excluded, a 92.9% agreement rate was obtained (Cassali et al., 2007). The same authors reported a sensitivity and specificity for the diagnosis of malignant tumors of 88.6% and 100%, respectively, and a sensitivity of 100% and specificity of 88.6% for the diagnosis of benign lesions. These studies did not correlate cytologic diagnosis with disease-free intervals or survival times, thus the use of cytologic criteria to accurately predict the biologic behavior of MGT is uncertain. Recent studies demonstrated that cells in malignant epithelial mammary tumors had significantly more irregular nuclear shapes that did control epithelial cells or cells in benign tumors based on differences in fractal dimension and on nuclear diameter and roundness. These morphometric parameters could help in the preoperative cytologic evaluation of canine mammary gland tumors (Simeonov, 2006a, Simeonov, 2006b).
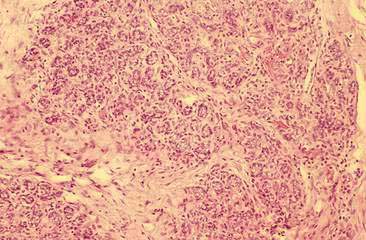
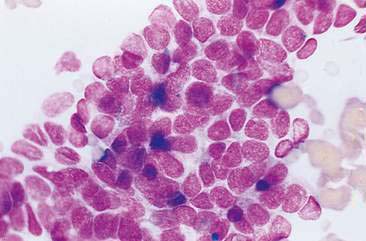
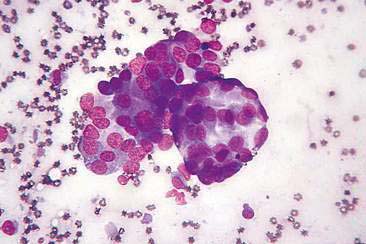
Cytologic examination of most mammary tumors reveals a background containing variable amounts of blood, basophilic proteinaceous material, lipid, and foam cells. Aspirates of benign epithelial tumors (adenomas and papillomas) typically reveal moderate to large numbers of epithelial cells arranged in sheets and clusters (Fig. 12-7). These cells are uniform in appearance with smooth nuclear chromatin and occasionally prominent, single, small, round nucleoli (Allison and Maddux, 2008). Acinar structures may be seen in samples from adenomas and palisade; papillary and trabecular cell arrangement can be observed in other benign epithelial tumors (Masserdotti, 2006). Benign complex adenomas or papillomas, fibroadenomas, and benign mixed tumors may yield sheets and clusters of uniform-appearing epithelial cells and individualized or clumped spindle-shaped cells of myoepithelial (complex tumors) or mesenchymal (mixed tumors) origin. Myoepithelial cells may also appear as oval free nuclei (Allison and Maddux, 2008). Examination of mixed mammary tumors may reveal the presence of cartilage or bone elements such as osteoblasts, osteoclasts, hematopoietic cells, and/or bright-pink extracellular material representative of osteoid (Fernandes et al., 1998) (Figs. 12-8 and 12-9). Mixed mammary tumors can be difficult to diagnose using exfoliative cytology. For instance, the presence of spindle-shaped cells may not be sufficient for diagnosis of mixed or complex tumors. Allen et al. (1986) have noted that spindle cells were identified in the mammary tumors evaluated in their study, yet the presence of these cells did not correlate significantly with histologic classification of complex or mixed tumors. Aspirates of mixed tumors also may not reveal all of the cells composing the tumor. In a case report, aspiration of a mammary mass in a dog revealed the presence of osteoblasts displaying moderate anisokaryosis and anisocytosis, osteoclasts, hematopoietic cells, and pink extracellular material (Fernandes et al., 1998). No epithelial cells were noted in the sample. Thus, the multiple differentials included benign or malignant mixed mammary tumor, osseous metaplasia, and osteosarcoma. Histopathology confirmed that the neoplasm was a benign mixed mammary tumor.
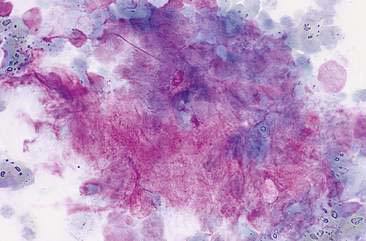
FIGURE 12-9 Mixed mammary tumor. Tissue aspirate. Dog. Clump of spindle-shaped cells associated with large amounts of extracellular pink material from the same aspirate shown in Figure 12-8. (Wright-Giemsa; HP oil.)
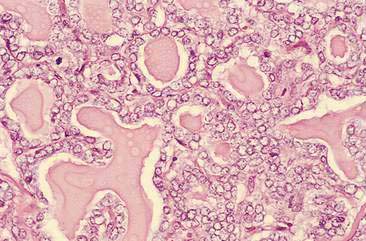
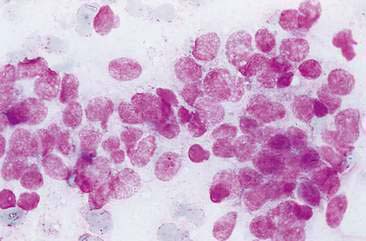
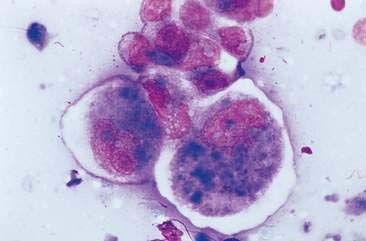
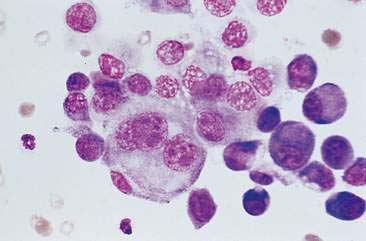
Malignant mammary tumors may be diagnosed based on the cytologic appearance of the cell types present and the observation of more than three criteria of malignancy. Adenocarcinomas are characterized by epithelial cells arranged in sheets (Fig. 12-10) and clusters, or sometimes individualized. Acinar arrangements (Fig. 12-11) may be observed (Masserdotti, 2006). The epithelial cells are typically round, with round to oval, eccentrically located nuclei and moderate amounts of basophilic cytoplasm that may contain amorphous basophilic secretory product and/or clear vacuoles (Allison and Maddux, 2008) (Fig. 12-12). Some of these vacuoles may appear as punctate vacuoles of variable number or as a diffuse clearing of the cytoplasm that distends the cell and displaces the nucleus peripherally. Criteria of malignancy that may be seen in these cells include increased nuclear-to-cytoplasmic ratio; moderate to marked variation in nuclear and cell size; nuclear molding; large, prominent, multiple, and/or abnormally shaped nucleoli; and binucleation and multinucleation. Increased mitotic activity and abnormal mitotic figures may be present (Figs. 12-13 and 12-14). Ductular carcinomas typically present with sheets and clusters of pleomorphic epithelial cells with high nuclear-to-cytoplasmic ratios and round, basal nuclei. These cells usually display more than three malignant criteria. Acinar structures, secretory product, and cytoplasmic vacuoles are not characteristic features of ductular carcinomas. Papillary and trabecular cell arrangements can be observed in malignant epithelial tumors (Masserdotti, 2006).
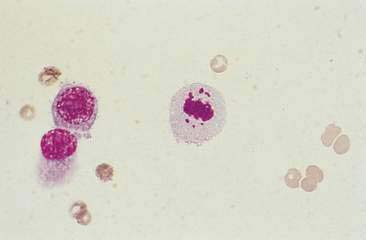
FIGURE 12-14 Mammary carcinoma. Tissue aspirate. Dog. Abnormal mitotic figure with lag chromatin from same specimen as shown in Figure 12-13. Lag chromatin results from abnormal formation of the mitotic spindle apparatus. Abnormal mitotic figures are considered one criterion of malignancy. (Wright-Giemsa; HP oil.)
Anaplastic carcinomas may present with very large, extremely pleomorphic epithelial cells occurring singly and in small clusters (Allison and Maddux, 2008). These cells tend to have bizarre nuclear and nucleolar forms. Multinucleation and abnormal mitotic figures are frequently seen. Inflammatory carcinomas, which are a locally aggressive form of mammary carcinoma, also present with large, pleomorphic epithelial cells exhibiting various criteria of malignancy as well as large numbers of nondegenerate neutrophils and macrophages (Lana et al., 2007). The cytologic appearance of inflammatory carcinoma may resemble mastitis. However, history, signalment, and presence of very anaplastic epithelial cells should be helpful for differentiation of these two conditions.
Squamous cell carcinomas of the mammary gland appear cytologically similar to those found in other body sites. The malignant squamous cells tend to occur individually or in small sheets. The nuclei may vary from small and pyknotic to large, round, and immature with prominent nucleoli. The nuclear-to-cytoplasmic ratio is variable and binucleation may be noted. The cytoplasm of the tumor cells is moderately to deeply basophilic (nonkeratinized) or may have a blue-green color characteristic of keratinization. Mammary squamous cell carcinomas may ulcerate, leading to the presence of inflammatory cells and phagocytized bacteria in the cytologic sample (Allison and Maddux, 2008).
Aspirates of malignant mixed mammary tumors may reveal epithelial cells and spindle-shaped, individualized cells of mesenchymal origin with one of these populations displaying nuclear and cellular criteria of malignancy. However, the presence of either population or predominance of one cell type over the other may depend on the area of tumor aspirated (Allison and Maddux, 2008). In carcinosarcomas, both epithelial and mesenchymal populations should display malignant features. Mammary sarcomas, such as osteosarcoma, fibrosarcoma, and liposarcoma, are of similar cytologic appearance to those found in other body sites. Sarcomas tend to exfoliate poorly, often resulting in samples of low cellularity. Depending on the type of tumor, pink extracellular material or lipid may be present in the background. In general, sarcomas are characterized by spindle-shaped to irregular cells arranged individually and in small clumps. The cytoplasm of these cells is moderately to deeply basophilic and the cytoplasmic borders tend to be indistinct. The cells display cytologic features of malignancy similar to those described for epithelial neoplasms.
Feline Mammary Gland Tumors
Mammary tumors are the third most common tumor in the cat, after hematopoietic neoplasms and skin tumors (Misdorp, 2002; Hayes and Mooney, 1985). The median age for MGT development in the cat is 10 years of age or older. Almost all (99%) of feline MGT occur in intact females (Lana et al., 2007). Domestic short hair and Siamese cats appear to have higher incidence rates (Hayes et al., 1981).
Development of feline MGT is thought to have a hormonal component. Intact females have an almost seven-fold greater risk of developing mammary neoplasms as compared to neutered females, and ovariohysterectomy has been reported to decrease the risk of MGT to 0.6% compared to intact females (Hayes et al., 1981). Regular, but not irregular, administration of exogenous progesterone was associated with a significantly increased risk of benign mammary tumors and mammary carcinomas in cats (Misdorp, 1991). Hormone receptor analysis has shown that normal feline mammary tissue contains estrogen and progesterone receptors in levels similar to those found in the dog (Millanta et al., 2005). However, unlike canine MGT, the majority of feline mammary neoplasms express very low levels of estrogen and progesterone receptors, which may be related to the high rate of malignancy found with mammary neoplasia in the cat. Other molecule targets have been investigated to elucidate prognosis or the pathways of tumorigenesis such as cyclin A, Cox-2 (Millanta et al., 2006b), HER2, VEGF and E-cadherin (Lana et al., 2007).
In contrast to the dog, the majority of feline mammary tumors are malignant with some studies reporting a greater than 80% incidence of malignant neoplasms (Hayes et al., 1981). Adenocarcinomas are the most prevalent malignant mammary tumor followed by carcinomas and sarcomas (MacEwen et al., 1984). Recently, secondary or postsurgical inflammatory carcinomas (Pérez-Alenza et al., 2004) and lipid-rich carcinoma (Kamstock et al., 2005) have been described, for the first time, in cats. Malignant MGT in cats tend to grow rapidly and metastasize to regional lymph nodes, lung, pleura, liver, diaphragm, adrenal glands, and kidneys (Lana et al, 2007). The single most important prognostic indicator for feline MGT is tumor size at time of diagnosis. Median survival times for cats with mammary tumors greater than 3 cm, between 2 and 3 cm, and less than 2 cm is 6 months, 2 years, and greater than 3 years, respectively (MacEwen et al., 1984). Thus, early diagnosis and treatment is very important for feline mammary malignancies.
The cytologic features of benign and malignant mammary neoplasms in the cat are similar to those described in the dog (see Figures 12-10, 12-13, 12-14). The reliability of cytologic criteria to differentiate between hyperplasia, benign tumors, and malignancies in the cat does not appear to have been reported (Baker and Lumsden, 1999). Given the high rate of mammary malignancy in cats, cytologic findings of a benign-appearing population of epithelial cells, particularly in an older cat with no history of progesterone administration, should be treated with some caution. In these cases, samples should be submitted for histopathologic examination to rule out the presence of a malignancy.
Treatment considerations will follow clinical and cytologic and/or histologic identification of a mammary neoplasm in a dog or a cat. If a malignancy is present, staging the extent of the disease should include three-view thoracic radiographs or CT scan of the lungs and any other potential metastatic sites as well as cytologic analysis of regional lymph nodes, metastatic lesions, and/or body cavity effusions. It has been proposed that treatment guidelines for malignant canine mammary gland tumors be based on tumor size, histopathologic type, and differentiation (Sorenmo, 2003). Surgical excision is the treatment of choice for both canine and feline mammary neoplasms. In dogs, it is recommended to perform ovariohysterectomy if intact in all malignant canine mammary gland tumors and to institute chemotherapy in an undifferentiated carcinoma in stage I (Sorenmo, 2003). There is limited information regarding efficacy of adjuvant therapy involving chemotherapeutics, radiation, or immune stimulation in canine and feline mammary malignancies. However, the combination of surgery and adjunctive doxorubicin chemotherapy resulted in improved long-term survival in cats with mammary gland adenocarcinoma (Novosad, 2003; Novosad et al., 2006). In addition, the combination of surgery and adjunctive 5-fluorouracil and cyclophosphamide chemotherapy demonstrated significant survival improvement in dogs with mammary gland carcinomas stage III/IV when compared with surgery alone (Karayannopoulo et al., 2001). In contrast, chemotherapy did not lead to an improved outcome in dogs with invasive malignant mammary gland tumors (Simon et al., 2006). The use of antiestrogens, such as tamoxifen, has been documented in a small number of clinical cases, with somewhat conflicting results in regard to tumor response. These drugs can be associated with undesirable estrogen-related side effects (Novosad, 2003).
Ovaries
Cytology is a valuable tool for diagnosis of ovarian tumors and ovarian cystic disease as recently demonstrated in a study with a diagnostic accuracy of cytology of 94.7% (Bertazzolo et al., 2004).
Special Collection Techniques
There is little information on ovarian cytology collection techniques. Ovarian biopsy is performed and surgical technique is well described elsewhere (Root Kustritz, 2006). Cytologic samples can be made by ultrasound-guided percutaneous fine-needle aspiration or intraoperatively during exploratory laparotomy.
Normal Anatomy and Histology
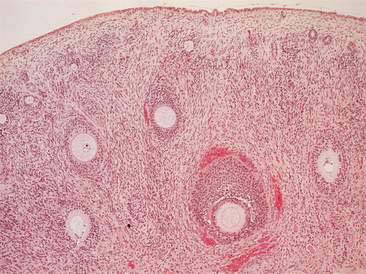
The ovary is composed of three broad embryologic origins: 1) the epithelium, which includes the outer layer lining (surface) epithelium of the modified mesothelium, the rete ovarii (remnants of the mesonephric tubules), and in the bitch, the subsurface epithelial structures; 2) the germ cells; 3) the ovarian stroma including the sex cords, which together contribute the endocrine apparatus of the ovary (MacLachlan and Kennedy, 2002). Each ovary lies within an ovarian bursa, an extension of the mesosalpinx, which is a fold of the peritoneum. Cuboidal epithelium called germinal epithelium covers the cortex of the ovary, and a layer of dense connective tissue, the tunica albuginea, is present underneath the epithelium. The canine ovary has small ingrowths of the ovarian surface that are called subsurface epithelial structures. The cortex of the ovary contains follicles, stromal connective tissue, and blood vessels. The ova develop in follicles that are of four types: primordial, primary, secondary, and tertiary. Each developing follicle has the oocyte, multiple layers of granulosa cells, and more peripheral thecal connective tissue cells (Fig. 12-15). Ovulation occurs when the follicle ruptures, releasing the ovum and allowing the space to fill with blood and luteal cells to form the corpus hemorrhagicum and the corpus luteum, respectively. In bitches and queens, cords of epithelial cells called interstitial glands, which are cells of an endocrine type, occur throughout the stroma. A medulla consisting of richly vascularized loose connective tissue, lymphatics, and nerves lies internal to the ovarian cortex. Channels lined by cuboidal epithelium called rete ovarii are present in this region (Foster, 2007).
The normal histology of ovaries from immature female dogs and sequential microscopic changes that occur during different stages of the estrous cycle are reviewed elsewhere (Rehm et al., 2007).
Normal Cytology
Cytology of normal ovarian tissue usually reveals small amounts of blood with no to moderate numbers of nucleated cells and moderate to large amounts of basophilic, proteinaceous material and clear lipid droplets. Normal ovaries are characterized cytologically by low to moderate numbers of one or more of the following cells based on the stage of the estrous cycle: adipocytes, individual fibrocytes/fibroblasts, small monolayered cohesive sheets of nonreactive mesothelial cells, granulosa cells that are uniform in size and shape and are arranged in acinar formations or in small loosely to cohesive aggregates, and singly luteal cells characterized by abundant pale basophilic cytoplasm with small, clear, discrete vacuoles and eccentric round to oval nuclei (Figs. 12-16, 12-17, 12-18, 12-19).
Cysts
Cysts in and around the ovary are a common finding during ovariohysterectomy in dogs and cats. There are two types of cysts: intraovarian and paraovarian. Intraovarian cysts include cystic rete ovarii, subsurface epithelial structure (only dog), vascular hematomas, and adenomatous hyperplasia of the rete ovarii (Foster, 2007; Klein, 2007).
Inflammation
Oophoritis, or inflammation of the ovary, is rare in domestic animals. Bacterial oophoritis occasionally is found in cats and dogs (Foster, 2007). The inflammation is around the ovary and within the uterine tube, suggesting that the causative bacteria ascended from uterus (Van Israel et al., 2002). In cats, feline infectious peritonitis can cause oophoritis.
Ovarian Neoplasia
Tumors of the ovary are uncommon in dogs and cats accounting for 0.5% to 6.3% of all canine tumors and 0.8% of all feline tumors (McEntee, 2002). The actual frequency of ovarian tumors may be underestimated as ovaries are not routinely sectioned at necropsy and are more commonly examined only if there is a gross lesion. In addition, the low frequency is affected by the fact that many companion animals are neutered at an early age. There are four main categories of ovarian tumors including epithelial, germ cell, sex cord–stromal, and mesenchymal. There are several other miscellaneous neoplastic diseases of the ovaries (mixed tumors and metastatic nonovarian malignant neoplasms). Clinical signs typically occur secondary to a space-occupying mass or to an effusion related to metastasis. Clinical signs in dogs with functional tumors secondary to excessive estrogen and/or progesterone production include signs of persistent estrus, pyometra, and bone marrow toxicity. Ovarian tumors can be an incidental finding at the time of ovariohysterectomy or necropsy (Klein, 2007).
Epithelial Tumors
Epithelial tumors include papillary adenomas/cystadenomas, papillary adenocarcinoma, cystadenocarcinoma, rete adenomas, and undifferentiated carcinomas (MacLachlan and Kennedy, 2002) and account for 40% to 50% of canine ovarian tumors. Fifty percent of malignant epithelial tumors metastasize by implantation or lymphatic or vascular invasion. These tumors occur in older female dogs with a median age of 10 to 12 years (McEntee, 2002). Epithelial tumors are extremely rare in cats (Klein, 2007).
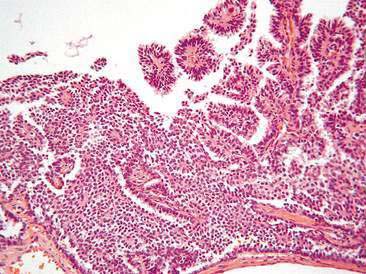
Papillary adenocarcinoma has been recently described cytologically. Cells are arranged in macro- to micropapillary forms (Masserdotti, 2006), acinar or tubular patterns, in cohesive clusters sometimes tridimensional, and occasionally as single cells (Figs. 12-20, 12-21, 12-22). Cells are round to polyhedral with a single oval nucleus. Nuclear chromatin is reticular to coarse. Nucleoli are indistinct to prominent single or multiple. Mild to marked anisokaryosis and anisocytosis are present. The cytoplasm is scarce to moderate and sometimes with finely discrete, clear vacuoles. Occasionally, large intracytoplasmatic vacuoles or signet ring cells are observed (Bertazzolo et al., 2004; Hori et al., 2006).
Sex-cordal Stromal Tumors
Sex-cordal stromal tumors include granulosa cell tumors, luteomas (also called interstitial gland, lipid, or interstitial cell tumors), and thecomas. In the dog, granulosa cell tumors account 50% of ovarian tumors and occur in elderly bitches with a median age of 10 to 12 years. Seventy-seven percent of granulosa cell tumors produce estrogens and/or progesterone and up to 20% are malignant. Granulosa cell tumor is the most common sex-cordal stromal tumor in older cats and more than 50% are malignant. Reported metastatic sites include peritoneum, lumbar lymph nodes, omentum, diaphragm, kidney, pancreas, spleen, liver, and lungs (McEntee, 2002). Granulosa cell tumors may be confused sometimes with ovarian epithelial tumors even in histological preparations. Useful immunohistochemical markers to distinguish these two tumors are cytokeratin 7 and inhibin-α. Ovarian epithelial tumors cells are positive to cytokeratin 7 and negative to inhibin-α while granulosa tumor cells and thecomas are negative to cytokeratin 7 and positive to inhibin-α (Riccardi et al., 2007; Klein, 2007).
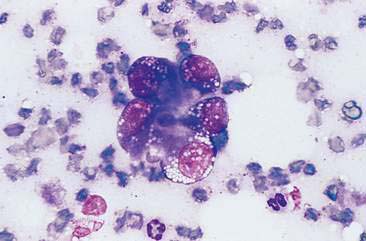
Cytologically, granulosa tumor cells are usually in monolayered, loosely cohesive clusters and often have acinar to tubular pattern (Fig. 12-23). Cells are arranged sometimes in an acinar pattern surrounding amorphous eosinophilic extracellular material called Call-Exner–like bodies. Capillary-like structures are occasionally evident inside large clusters of cells (Fig. 12-24). Single cells appear from round to polyhedral. Nuclei are round to oval with indistinct nucleoli and mild to moderate cellular atypia. The cytoplasm is scarce to moderate with variable amounts of vacuolated cytoplasm (Bertazzolo et al., 2004).
Feline luteomas have been recently cytologically described. Large round to oval cells arranged individually or in loose clusters are observed. Nuclei are central to eccentric with granular chromatin with prominent, small, central nucleoli. Anisokaryosis is mild to moderate. Cytoplasm is lightly basophilic with many variably sized clear vacuoles and occasionally small purple granules (Choi et al., 2005).
Germ Cell Tumors
Germ cell tumors include dysgerminoma (counterpart of the testicular seminoma), teratoma, and teratocarcinoma. Dysgerminoma represent a less-differentiated tumor than mature teratoma. Germ cell tumors comprise 6% to 20% of canine ovarian neoplasms and 15% to 27% of feline ovarian neoplasms. The median age of dogs with dysgerminoma is 10 to 13 years and with teratomas is 4 years. The age of cats that have been reported to have dysgerminomas ranges from 1 to 17 years with a median of 5 years. Metastasis is reported to develop in 10% to 20% of canine dysgerminomas with regional lymph nodes, liver, brain, and kidney as the primary sites. Young cats (5 to 8 months) and dogs have teratomas (McEntee, 2002; Klein, 2007).
Dysgerminomas are seen cytologically as a predominant population of markedly pleomorphic, large, round to polygonal cells arranged singly or in loose aggregates. Cells range from 20 to 70 μm of diameter. Nuclei are large and round to oval with chromatin stippled to reticular (Figs. 12-25 and 12-26). Nucleoli are prominent multiple and of variable shape and size. Aberrant mitotic figures and bi- or multinucleated cells are commonly noted. Anisocytosis and anisokaryosis are marked. The cytoplasm is scant clear to blue-gray with variably distinct margins. Occasionally eosinophilic, granular, intracytoplasmic material is noted. Small lymphocytes can be observed (Bertazzolo et al., 2004; Brazzell and Borjesson, 2006).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree