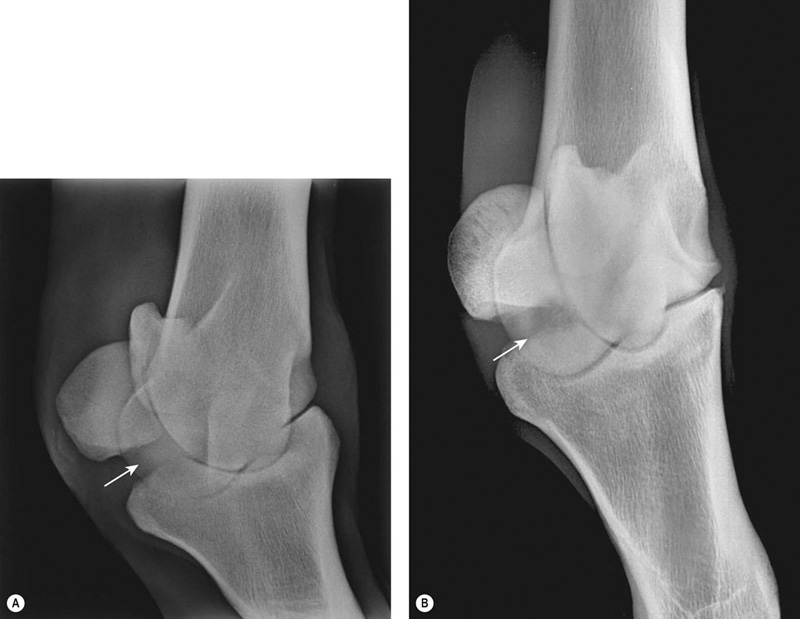
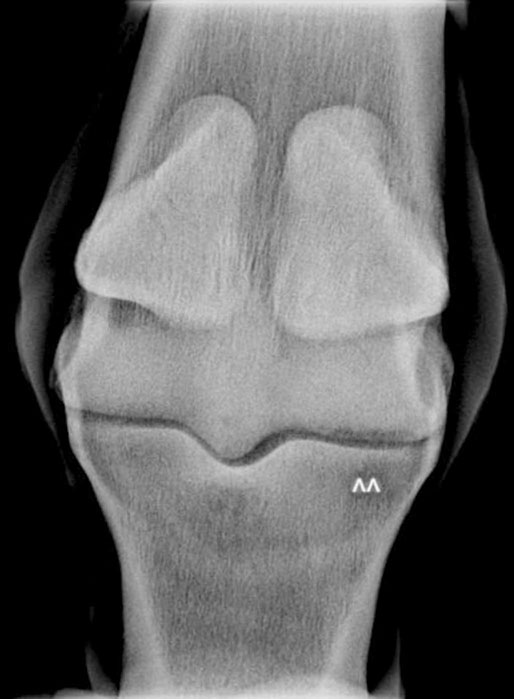
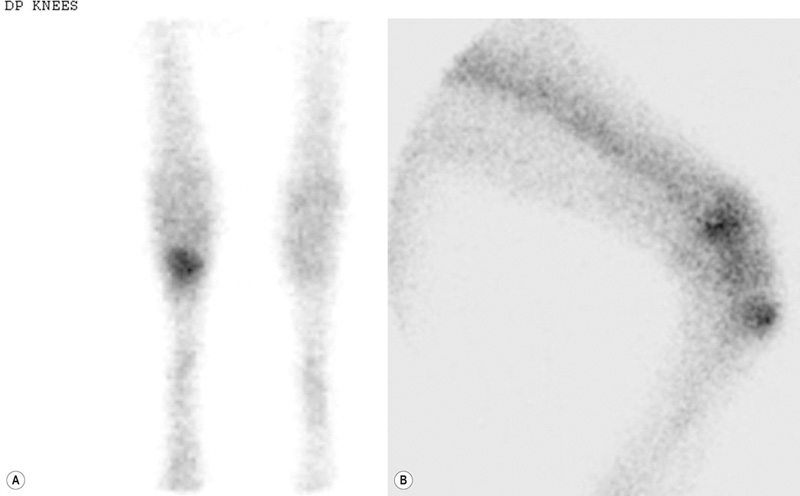
Bone injuries are common among racehorses. Catastrophic fractures account for over 80% of all racecourse fatalities.1 Stress fractures lead to significant loss of training days and disease of subchondral bone affects a high proportion of horses, often leading to permanent impairment of joint function and consequent loss of performance and premature retirement.2 A large body of evidence has accumulated over the last 20 years to demonstrate that the majority of these injuries are the end result of a progressive process associated with fatigue damage of the bone. In order to achieve the best outcomes for racehorses, clinicians need to have a good understanding of bone physiology and pathology and the reader is encouraged to study Chapter 8 for a review of relevant information. In addition, clinicians must have a working knowledge of the types of lesion that arise, their specific anatomical locations and the subtle radiographic and ultrasonographic features that may be associated with them. While pathological changes in subchondral bone have been characterized in numerous studies, there is no unifying hypothesis to explain how they culminate in clinical lesions. We speculate the following may happen: Increased volume fraction of subchondral bone (due to infilling of trabecular spaces with new bone and which may be apparent radiographically as ‘sclerosis’) in response to higher loads associated with greater exercise, reflects an adaptive response. The resultant structure will be stronger but the more dense bone will be less compliant and, therefore, more prone to injury during impact loading. In the face of a continued cyclical burden, injury may manifest in the form of microdamage and/or osteocyte necrosis. The matrix will become hypermineralized following osteonecrosis, making it more brittle and, therefore, further prone to damage. Disruption of blood supply following more extensive matrix damage exacerbates the rate of osteonecrosis. At some stage (e.g. following a period of reduced loading) a remodeling response to remove damaged or necrotic bone is initiated. This may result in a focal defect in subchondral bone that acts as a stress-riser, which predisposes to fracture. Alternatively, if a relatively large volume of subchondral bone is affected, a void may be created between vital and necrotic tissue during the initial resorptive phase of remodeling. On resumption of loading the superficial, necrotic tissue may collapse into the void, resulting in a depression in the joint surface or may fracture away from the parent bone, leaving a deep ulcer in the joint surface (Fig. 22.1). Longitudinal studies with suitable imaging techniques are required to substantiate this hypothesis. It is vital that a properly positioned third carpal skyline projection (flexed dorsal 60° proximal-dorsodistal oblique) is included in radiographic examination of the carpus to demonstrate structural changes associated with this syndrome. While easy to accomplish with practice, this projection is often poorly executed and, therefore, significant abnormalities are overlooked. The lesions are most commonly visualized in the radial articular facet of the third carpal bone but can also occur in the intermediate facet. The radiographic appearance of the bone progressively changes as the inter-trabecular spaces are filled with new bone, resulting in an amorphous radiopaque ‘haze’ as the normal trabeculated pattern of the bone is steadily lost. As the disease advances, focal areas of resorption, often associated with nutrient foraminae, appear within the sclerosis3 (Fig. 22.2). Fissure fracture of the dorsal cortex may become visible and in advanced cases, sagittal or curvilinear slab fracture lines may be visualized. On scintigraphic examination, moderate increased radiopharmaceutical uptake (IRU) is visualized over the entire outline of the third carpal bone and this will become more intense and focal in the event of fracture (Fig. 22.3). Progressive disease of the bone underlying the articular surface of the mid palmar/plantar aspect of the condyles of the third metacarpal and third metatarsal bones is extremely common in Thoroughbred racehorses.2,4,5 Originally termed ‘traumatic osteonecrosis’, it has more recently been referred to as ‘palmar osteochondral disease’.2 Lesions arise at the point of articulation with the basilar half of the proximal sesamoid bones when the joint is at full extension and are presumed to be due to excessive, repetitive stress at these points. A spectrum of pathology is seen, from faint bruising of subchondral bone, visible through the articular cartilage, to deep ulcers associated with saucer-shaped complete, displaced fractures of a 2–3 mm depth of articular cartilage and subchondral bone5,6 (Fig. 22.4). It is assumed that the different degrees of pathology represent varying stages of the same process. In addition, lameness in which the only related findings are pain localized to the distal condyles of the third metacarpal or metatarsal bone, associated with IRU in the palmar/plantar condyles of these bones is extremely common. It is likely that this is part of the same syndrome. Lesions are more common and progress to a greater extent in the medial condyles of the third metacarpal bone and lateral condyle of the third metatarsal bone.7 However, the clinical condition commonly occurs bilaterally or quadrilaterally and this is reflected in the pattern of gait abnormality seen. Associated lameness is typified by a shortened protraction of each limb at the trot, a low limb flight and a lack of animation or ‘bounce’ in the gait. Low limb flight and increased pelvic excursion seen with bilateral metatarsal disease often leads to the horse catching its toes, giving a characteristic wear pattern to the feet. Radiography is unrewarding at early stages of the disease. Attempts to demonstrate sclerosis of subchondral bone in the palmar or plantar aspect of the condyles is hampered by superimposition of other radiopaque structures. Dorsal 45° proximal 45° lateral-plantarodistomedial oblique and the opposite oblique projections8 provide a relatively unobscured image of a small area of each respective condyle and can demonstrate structural changes associated with increased bone volume fraction in some cases (Fig. 22.5). In more advanced cases of disease that are associated with defects in the subchondral bone plate created by collapse or ulceration of the subchondral bone, crescent shaped radiolucent defects can be identified on lateromedial or flexed dorsopalmar/plantar projections (Fig. 22.6). In these end stages of palmar osteochondral disease the joint surface can collapse or sustain a saucer fracture, leading to severe pathology of articular cartilage (Fig. 22.4). This almost always results in obvious signs of inflammation of the affected joint and is associated with profound lameness.
Repetitive strain injuries of the skeleton in high performance equine athletes
Introduction
Clinical syndromes associated with repetitive strain injury of bone
Recurrent strain injury of subchondral bone associated with synovial articulations
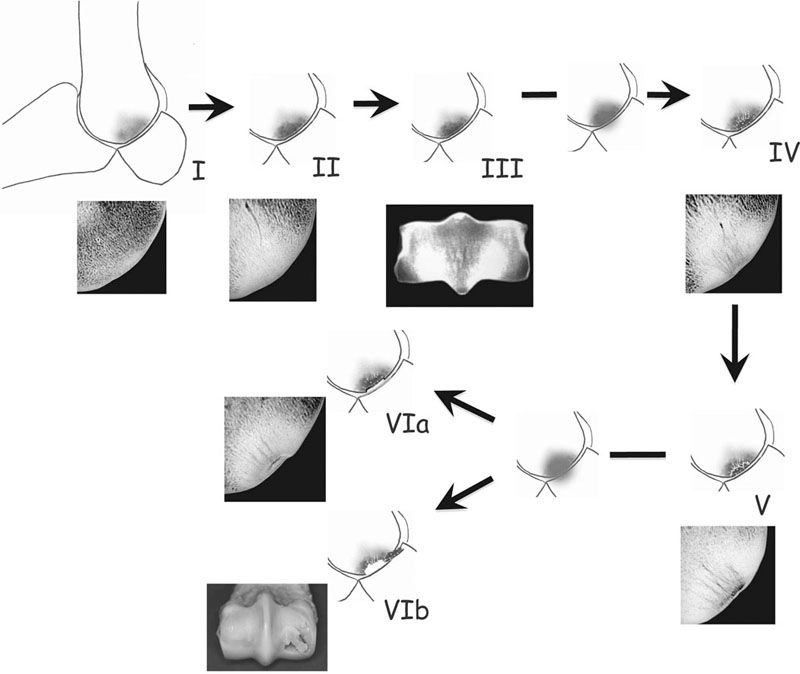
I – relatively open, porous trabecular structure in a young horse that has undertaken minimal work; II & III – with exercise new bone forms on the trabeculae, progressively filling the vascular spaces and increasing apparent density (volume fraction) of the bone; IV – further exercise (and impact loading of the brittle bone volume) results in death of osteocytes and hypermineralization of bone tissue and early in-growth of new blood vessels; V – recruitment and activation of osteoclasts and focal resorption of damaged bone at the interface between living and dead bone (probably accelerated by a period of low strain – rest). Further intense exercise in the face of the localized porosity leads to either: VIa – collapse of more superficial, dead subchondral bone into the cavity, resulting in a depression at the articular surface, or: VIb – saucer fracture of undermined segment of subchondral bone. Microradiographs courtesy of Professor Alan Boyde, Barts and The London School of Medicine and Dentistry, Queen Mary University of London.
Subchondral bone disease in the third carpal bone
Recognition
Diagnosis
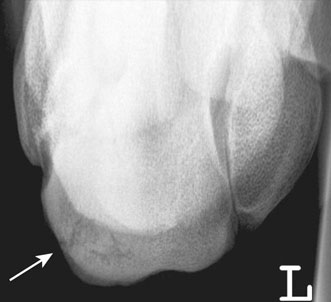
Subchondral bone disease of the distal condyles of the third metacarpal and third metatarsal bones
Recognition
Presentation
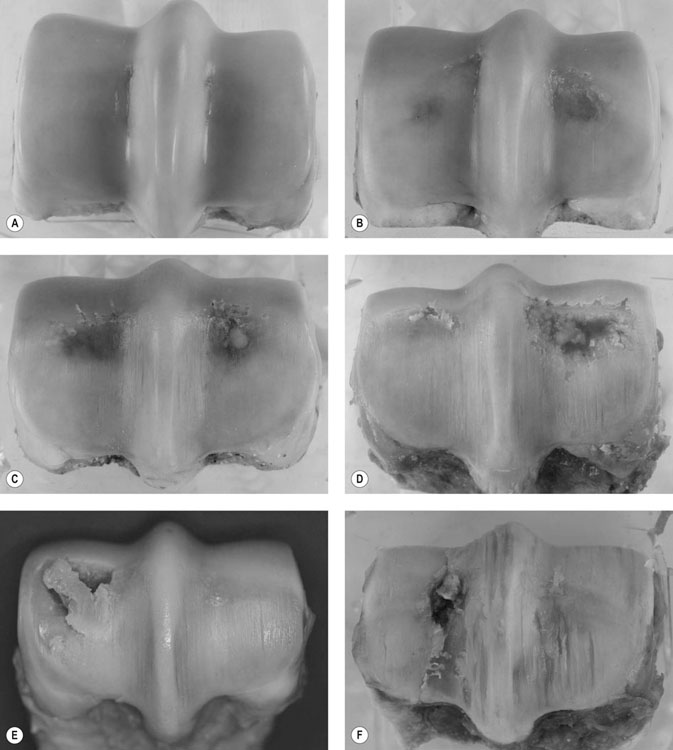
(A) Six-year-old gelding; lateral to the right. Normal appearance to subchondral bone in both condyles but faint linear fissures visible in medial and lateral condylar grooves. (B) Seven-year-old gelding; lateral to the right. Bruising of subchondral bone visible through intact overlying articular cartilage. Grade I palmar osteochondral disease (POD) lesion medial condyle, Grade II POD, lateral. There is a small linear fissure in the medial condylar groove. (C) Seven-year-old gelding, lateral to the right. Grade II POD lateral condyle and Grade I POD medially. There is localized fibrillation of articular cartilage in the region of the transverse ridge in both condyles. There are multiple, marked wear lines in the articular cartilage overlying both condyles and the sagittal ridge. (D) Seven-year-old gelding, lateral to the left. Grade III POD medial condyle associated with severe disruption of the articular surface with complete ulceration of articular cartilage and a deep cavity in the underlying subchondral bone, which is filled with fibrin. Focal erosion and tearing of articular cartilage overlying the transverse ridge in the lateral condyle. There are multiple, marked wear lines in the articular cartilage overlying both condyles and the sagittal ridge.
(E) Five-year-old gelding, lateral to right. Grade III POD medial condyle associated with incomplete saucer fracture of superficial bone and articular cartilage overlying a large defect deeper in the subchondral bone (note that the articular cartilage overlying the fracture fragment is intact). Grade I POD lateral condyle. There are multiple, marked wear lines in the articular cartilage overlying both condyles and the sagittal ridge. (F) Four-year-old gelding. Lateral to the left. Complete lateral condylar fracture (one month after surgical repair) associated with a Grade III POD lesion lateral condyle. There are multiple, marked wear lines in the articular cartilage overlying both condyles and the sagittal ridge.
Diagnosis