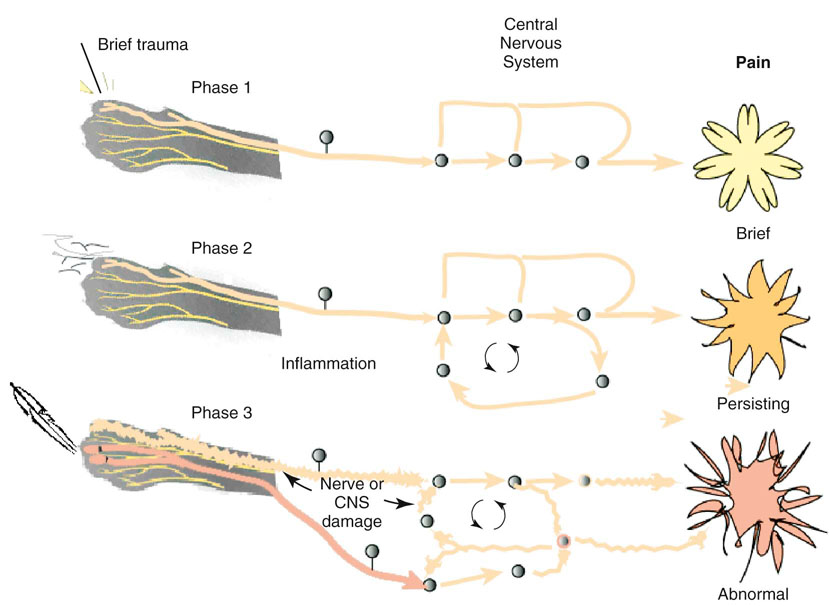
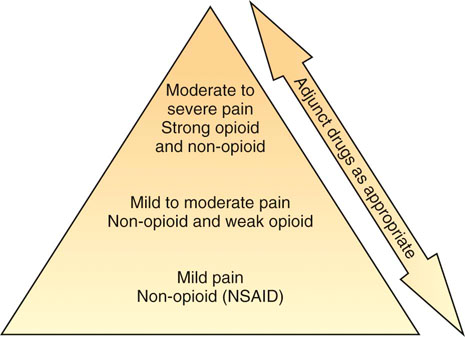
Steven M. Fox and Robin Downing Although pain in canine patients is inevitable in the face of surgery, trauma, injury, osteoarthritis, and so on, suffering is optional. It is well established that uncontrolled pain is not only ethically problematic, but physiologically damaging as well.1 In recent years pain management modalities have been developed in human medicine to address the evolving understanding of the complexities of the pain experience. Many of these pain treatment strategies have been imported into veterinary medicine. Pain and suffering should be sought out, the cause identified to the greatest degree possible, and alleviated when feasible. • Unnecessary suffering for the painful patient • Tissues that are unable to heal normally • Restriction of range of motion and other tissue dysfunction from the influence of pain signals • Undermining the trust inherent in the family–pet relationship • Tissue contraction/atrophy from disuse driven by pain • Resentment on the part of the patient of all caregivers • The transition/transformation from adaptive pain that serves a purpose to a maladaptive pain state in which the pain itself is pathologic An underlying tenet of veterinary medicine is “Do No Harm,” and this applies equally to physical rehabilitation. Fundamental to the success of any physical rehabilitation regimen is the control of pain. And fundamental to the success of controlling pain is the understanding that a multifaceted (also called multimodal) approach appears to provide optimal outcome. The goal for successful integration of pain management and physical rehabilitation is to build a pain management plan that allows for maximum comfort to open the door for maximum movement. There are excellent sources for in-depth discussions of pain physiology and pathophysiology.2–4 Within the context of physical rehabilitation it is important to have an understanding of basic pain processes, as this information will influence treatment choices for individual patients. For instance, patients experiencing acute pain following orthopedic surgery have different needs than the elderly dog experiencing the chronic maladaptive pain associated with long-standing osteoarthritis. Practitioners must appreciate the differences among patients and their pain experiences so that they may create individualized pain management strategies. Likewise, practitioners must understand properties unique to different drugs and drug classes as well as these drugs’ mode of action to create a targeted approach to pain.5 Pain can be divided into two general categories recognized as adaptive and maladaptive. Adaptive pain protects the body from injury and promotes healing by inhibiting activity when injury has occurred. In contrast, maladaptive pain reflects pathologic activity of the nervous system—the result of complex processes of neuronal plasticity—pain as a disease state.5,6 Adaptive, everyday acute pain (Figure 14-1) is also known as nociceptive or physiologic pain and occurs when noxious stimuli (mechanical, thermal, or chemical) are transduced into electrical signals that are then transmitted to the spinal cord. These signals undergo modulation in the spinal cord before being relayed to the brain, where the conscious brain interprets these transmissions, resulting in pain perception. Adaptive pain is purposeful. Dr. Frank Vertosick states, “Pain is a teacher, the headmaster of nature’s survival school.”7 Acute pain tends to be of relatively short duration and, if addressed properly, is relatively easy to treat. Chronic pain was traditionally defined as pain lasting more than 3 or 6 months, depending on the source of the definition.8,9 More recently, chronic pain has been defined as “pain that extends beyond the period of tissue healing and/or with low levels of identified pathology that are insufficient to explain the presence and/or extent of pain.”10 There is no general consensus on the definition of chronic pain. In clinical practice it is often difficult to determine when acute pain has become chronic—when adaptive pain crosses the threshold to become maladaptive. A progression from adaptive to maladaptive pain can be described as a spectrum composed of three major stages or phases of pain, suggesting that different neurophysiologic mechanisms are involved, depending on the nature and time course of the originating stimulus.11 These three phases are (1) the processing of a brief noxious stimulus, (2) the consequences of prolonged noxious stimulation, leading to tissue damage and peripheral inflammation, and (3) the consequences of neurologic damage or changes, including peripheral neuropathies and central pain states. This spectrum of architectural change within the nervous system and the resultant pain pathology may be represented graphically as in Figure 14-2. The journey of the nervous system toward maladaptive pain is often described as windup. Windup describes activity-dependent plasticity characterized by a progressive increase in action potential output from dorsal horn neurons elicited during the course of prolonged, repeated low-frequency C-fiber or nociceptor stimuli.12,13 One receptor critical to this process is the N-methyl-D-aspartate (NMDA) receptor. NMDA receptors are only activated in the face of prolonged depolarization such as occurs with chronic pain.14 Their activation can cause neural cells to sprout new connective endings,15 thus expanding the receptive fields of the painful patient. Accompanying windup, less glutamate is required to transmit the pain signal and more antinociceptive input is required for analgesia. The complexity of maladaptive pain and the phenomenon of windup continues to provoke ongoing study. Studies have implicated activated microglia, norepinephrine (NE) levels, serotonin (5-HT) activity, as well as transient receptor potential (TRP) receptors as playing a role in maladaptive pain. Pain memories imprinted within the CNS, mediated by NMDA receptors, produce hyperalgesia and contribute to allodynia—pain that is produced by a stimulus that does not normally provoke pain. A preoperative blockade of nociception may prevent postsurgical wound pain or pain hypersensitivity following surgery. This is the concept of preemptive analgesia articulated by the eminent pain physiologist Patrick Wall.16 In 1988, Professor Patrick Wall introduced the concept of preemptive analgesia to clinicians with his editorial in the journal Pain.17 Successful preemptive analgesia must meet three criteria. It must be (1) intense enough to block all nociception, (2) wide enough to cover the entire surgical area, and (3) prolonged enough to last throughout surgery and even into the postoperative period. General anesthesia with an inhalant agent (e.g., isoflurane, sevoflurane) does not prevent central sensitization. The potential for central sensitization exists even in unconscious patients who are unresponsive to surgical stimuli.18,19 Noxious stimuli still enter the spinal cord during apparently adequate anesthesia.20 The purpose of preemptive analgesia is to prevent sensitization of the nervous system throughout the perioperative period. Pain is to be expected from an initial surgery and the hypersensitivity that subsequently develops. Analgesia administered after sensitization may decrease pain somewhat, but has little long-term benefit in addressing the pain resulting from postsurgical inflammation. Analgesia administered before surgery limits inflammatory pain and decreases subsequent hypersensitivity. The most effective preemptive analgesic regimen occurs when it is initiated before surgery and continued throughout the postoperative period. Equally important is to use a complement of medications that work synergistically; for example, an opioid, alpha-2 agonist, NMDA antagonist, local anesthesia, and an NSAID.21–23 It bears reminding that good nursing care in the immediate postoperative period can contribute to the comfort of the surgical patient.24 For the majority of rehabilitation patients that have experienced surgery, NSAIDs form the foundation of pain management in the postoperative period. Antiinflammatory drugs play such a substantial role in perioperative pain management25 because surgery cannot be performed without subsequent inflammation. Reducing the inflammatory response in the periphery, and thereby decreasing sensitization of the peripheral nociceptors, should attenuate central sensitization.26 In human medicine, the use of perioperative NSAIDs has reduced the use of patient-controlled analgesic morphine by 40% to 60%.27,28 Depending upon the patient’s needs, an NSAID may be adequate as a stand-alone pain management agent. However, the rehabilitation practitioner must be especially vigilant about pain issues in the immediate postoperative period. Inadequately managed postoperative (adaptive) pain can transition to maladaptive pain, creating additional problems for the physiotherapy patient. A postoperative rehabilitation patient should be assessed for pain regularly, both at the time of therapy and through conversation and questioning with the pet owner to evaluate activities of daily living. One source of methods for scoring and evaluating pain may be found in the AAHA/AAFP Pain Management Guidelines for Dogs and Cats.29 It may be that a postsurgical patient may need one or more of the adjunctive pain management strategies outlined in the following chronic pain section. When building a comprehensive pharmacologic pain management plan for these patients, it is instrumental to consider the World Health Organization (WHO) Cancer Pain Ladder as a model (Figure 14-3). The principle articulated in this model is to begin pain management using medications that are effective against mild to moderate pain—generally NSAIDs—and building upon that foundation by adding adjunctive medications that attack maladaptive pain via synergistic pathways/modes of action as well as nonpharmacologic strategies. The result is a pyramid in which each addition builds upon the last layer of pain management, providing a foundation for the next layers to be added. The WHO ladder for treating cancer pain in humans30 has been adopted for a variety of different types of pain in both humans and veterinary patients. As veterinary pain management becomes more sophisticated, pharmacologic combination therapies from human medicine are being considered and implemented. It is much easier to identify maladaptive pain states in humans than in nonhuman species. Figure 14-4 suggests a logical approach to managing the progressive pain state, wherein pain management uses a multimodal scheme in which drug classes are added rather than substituted. The doses are empiric, and one could debate the order of implementation from bottom to top. The merit of this approach is the addition of agents from different drug classes, attempting to block as many of the pain pathways (transduction, transmission, modulation, and perception) as possible. The illustration also acknowledges the important role of nonpharmacologic contributions to pain management. Care must be taken as clinicians await clinical trials that clearly and specifically provide information about (1) each drug’s mode of action, (2) the pharmacokinetics in the target species, and (3) the physiology as well as pathophysiology in the veterinary target species. At the moment, only a handful of studies may be cited in support of the use of several of the adjunctive agents that are enjoying increasing use in veterinary patients.31–34 Pretreatment screening should include a thorough physical examination, including a review of the patient’s medical history and medical record for any previous or concurrently administered medications. A metabolic profile to evaluate renal and hepatic function and a hemogram are fundamental. A more sensitive way to assess for potential renal compromise is to include a test for microalbuminuria35—a marker for increased permeability of the basement membrane of the glomerulus. Although not pathognomonic for chronic renal failure, microalbuminuria alerts the practitioner to the need for additional vigilance of renal function. There is no consensus regarding the frequency of monitoring metabolic and organ function in the face of chronic-use medications for pain; however, a 2- to 4-month interval for monitoring is reasonable in most older dogs. Nonsteroidal antiinflammatory drugs remain the cornerstone for treating pain associated with degenerative joint disease (osteoarthritis; OA) and other sources of chronic pain. Nonsteroidal antiinflammatory drugs have effects at numerous locations along nociceptive pathways, both peripherally and centrally. This class of drugs manifests antiprostaglandin effects peripherally, but they are also considered central-acting because of their inhibition of cyclooxygenase within the dorsal horn of the spinal cord. Cyclooxygenase-2 (COX-2)–mediated PGE2 production contributes to a late-onset, prolonged, and diffuse phase of central sensitization.36 Cyclooxygenase-2-specific NSAIDs dampen central sensitization, and therefore play an important role in a multimodal pain management strategy. Cyclooxygenase-1–sparing NSAIDs (Deracoxib,37 meloxicam, and firocoxib38) have their optimal theoretical advantage where there is concern for platelet aggregation, or homeostasis. The integration of an NSAID in a pain management protocol is particularly appealing when considering the theory by some39 that the origin of all pain is inflammation and the inflammatory response. However, it must be appreciated that the ceiling effect of NSAIDs can limit their analgesic potential, thereby suggesting they are best, when used alone, for mild and moderate pain. Among a population of canine pain patients, all NSAIDs are effective, but a practitioner may witness variable responses to NSAIDs among individuals.
Rehabilitating the Painful Patient
Pain Management in Physical Rehabilitation
Pain Processes
Adaptive and Maladaptive Pain
Adaptive to Maladaptive Pain
Putting Together a Pain Management Plan for Surgery Patients
Postsurgical Pain and Rehabilitation
Preemptive Analgesia
Postoperative Analgesia
The Elements of a Comprehensive Pain Management Plan for Chronic Pain Patients
Pharmaceutical Pain Management for the Maladaptive Pain Patient
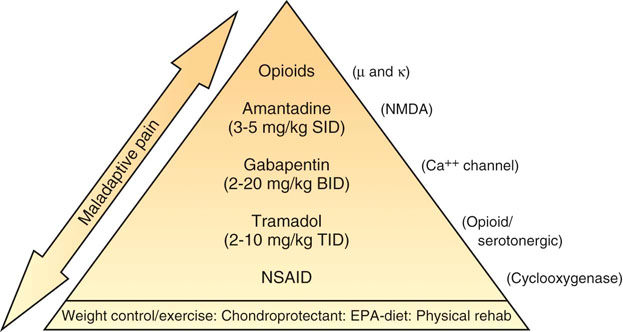
Figure 14-4 Proposed analgesic ladder for progressing pain states using drugs with different (complementary) modes of action. (Doses are empiric, and combination efficacy and safety data are unexplored.)
Nonsteroidal Antiinflammatory Drugs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree