Chapter 24
Ranavirus
Ranaviruses are members of Iridoviridae and are known to cause morbidity and mortality in amphibians, reptiles, and fish. Current research is exploring the mechanism of interclass transmission and variations in host susceptibility, both of which can potentially contribute to mortality events in nature. Given that ranaviruses in reptiles are discussed elsewhere (see Chapter 5) and that fish are not a focus of this book, this chapter will only address ranaviruses in amphibians.
Ranaviruses are globally distributed, having been reported on five continents.1 Anthropogenic spreading (e.g., from infected animals used as live fishing bait, field and recreational equipment, boots, and import/export of infected animals for food or pets) has resulted in exposure of naïve populations and subsequent mortality events in wild amphibians.2–4 Ranaviruses have infected 14 families and more than 70 individual species of amphibians and, likely, can infect most if not all amphibian species.1 Host–pathogen coevolution may have resulted in widespread infection with minimal health effects in intact ecosystems, but these same ranaviruses may be virulent in nonadapted amphibian species or in adapted species that are in degraded environments and subject to stressors.1,2,5
Susceptibility to ranaviruses varies by host species, ranavirus isolate, and across phylogenetic lineages.5,6 Eggs appear to be resistant to infection, likely because of the mechanical protection of the capsule.7 In general, hatchling and metamorphs are the most susceptible age groups7; however, adults are reported most often in die-offs of several European species.8–10
Ranaviruses are horizontally transmitted via direct contact, ingestion of virus or infected animals, and water exposure.11 Vertical transmission is suspected but remains unknown.11,12 There is evidence for interclass transmission among amphibians, reptiles, and fish.13–19 Therefore, community composition may play a role in emergence within an ecosystem.
Ranaviruses cause necrosis of many different cell types, leading to organ failure and death in susceptible individuals. Clinical signs include swelling (appendages, body), hemorrhages (especially on the ventrum and legs), ulceration, discoloration of the skin, lethargy, buoyancy problems, and erratic swimming (Figure 24-1).1 Additional gross lesions noted at necropsy may include hemorrhages of internal organs, tan friable organs, hydrocoelom (edema), and swelling of the lymph sacs (Figure 24-2). Microscopic lesions may include hemorrhage, cellular necrosis (e.g., hematopoietic tissue, hepatocytes, epithelial cells, endothelial cells), and inclusion bodies (intracytoplasmic; however, intranuclear have been reported but are rare [Figure 24-3]).1 In addition, caudates may develop ulcers on their feet, particularly the toe tips, and white polypoid lesions (noted in experimentally infected tiger salamanders).20 However, not all individuals will have observable ranaviral lesions, and sudden death may be the only sign. Incubation time remains unknown but may vary by host species, host age group, and virus isolate. The incubation period can be as short as 3 days but may also extend into weeks.21 In die-offs, ongoing mortality may occur in a population over a span of weeks to months, but later deaths are often attributed to secondary invaders.1 Finally, subclinical infection has been detected in wild amphibians22 and in experimental challenges.21,23 Individuals that are subclinically infected often have nonspecific histologic changes such as vacuolation of renal tubular epithelium and hepatocytes.
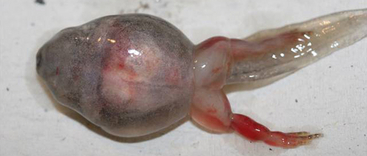
FIGURE 24-1 A Wood Frog (Lithobates sylvatica) larva with ranaviral disease has swelling of the body and legs and hemorrhage of the right rear leg.

FIGURE 24-2 Internal lesions in ranaviral disease can include hemorrhage and edema as seen in the exposed intestinal tract of this Green Frog (Lithobates clamitans) larva. (Photo courtesy Nathan Haislip, Wetlands Program, The University of Tennessee-Knoxville.)
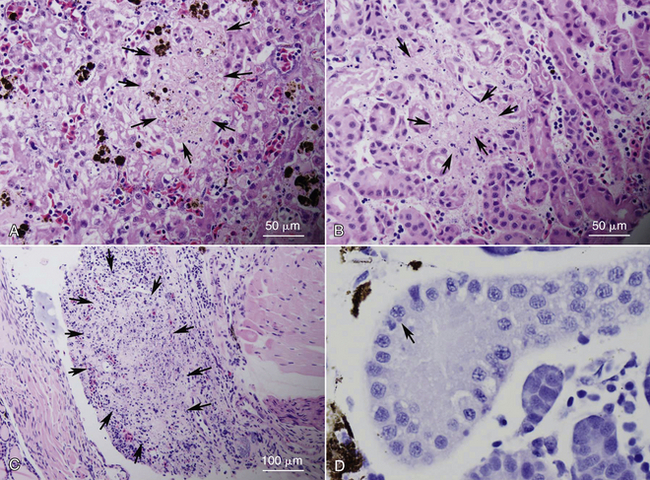
FIGURE 24-3 Examples of histopathologic changes observed in ranaviral disease. Necrosis of multiple organs (arrows), including liver (A), mesonephros (B), and lymphoid tissue (C) was observed in American Bullfrog (Lithobates catesbeianus) metamorphs from a die-off event. D, Cytoplasmic inclusion bodies (arrow) are not consistently seen but were observed within tubule epithelial cells of the mesonephros of an experimentally infected Wood Frog (Lithobates sylvatica) larva.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


