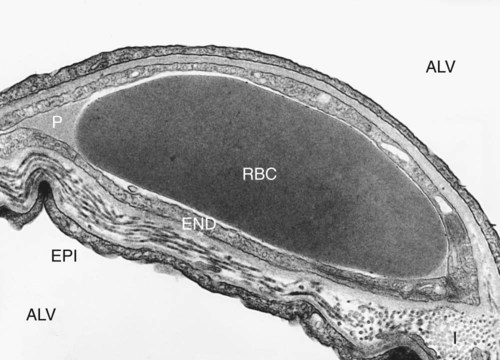
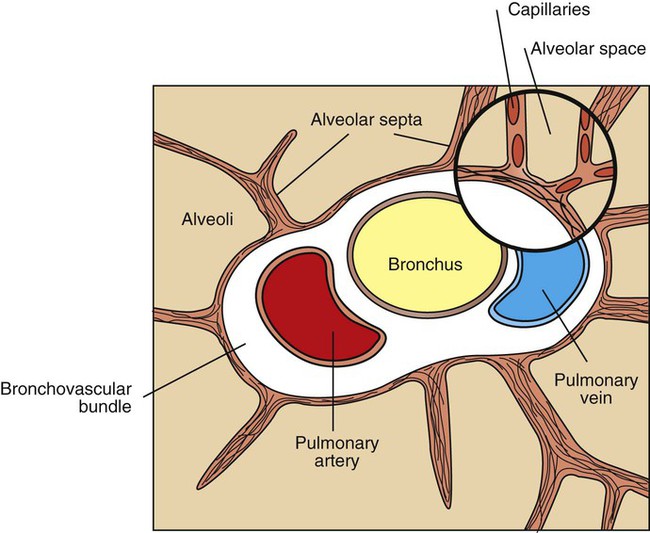
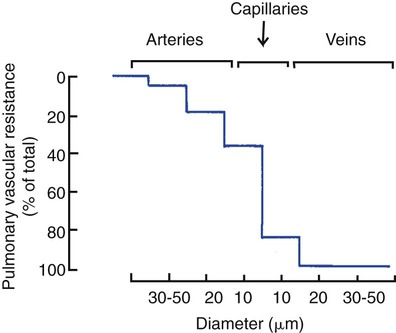
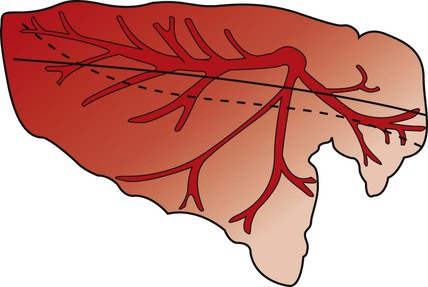
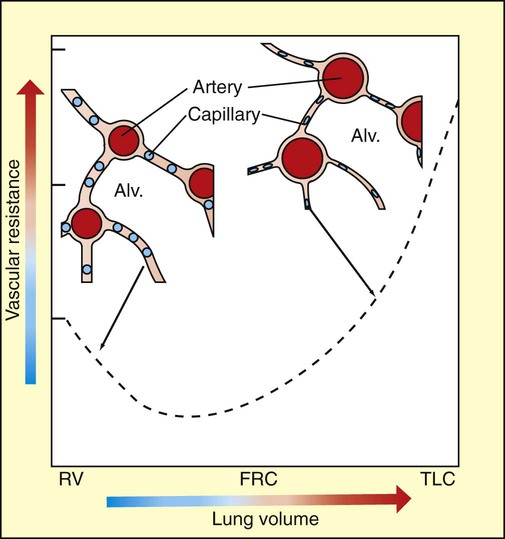
1. The structure of the small pulmonary arteries varies among species. 2. Functionally, pulmonary blood vessels can be classified as alveolar and extra-alveolar vessels. 3. The pulmonary blood vessels offer a low resistance to flow. 4. The distribution of pulmonary blood flow within the lung is influenced by several factors. 5. Passive changes in vascular resistance result from changes in vascular transmural pressure. 6. Neural and humoral factors cause contraction of the muscular pulmonary arteries. 7. Alveolar hypoxia is a potent constrictor of small pulmonary arteries. 8. During exercise the pulmonary circulation must accommodate a large increase in blood flow. 1. The bronchial circulation provides a blood supply to airways, large vessels, and, in some species, the visceral pleura. Alveolar vessels are the thin-walled capillaries that perfuse the alveolar septum (Figure 46-1). They are exposed almost directly to the pressure changes that occur in the alveoli during breathing. Extra-alveolar vessels include the pulmonary arteries, and veins, which occur together with bronchi in a loose connective tissue sheath called the bronchovascular bundle. This bundle is bounded by a limiting membrane to which alveolar septa are attached (Figure 46-2). The behavior of extra-alveolar vessels is determined by pressure changes within the connective tissue space of the bronchovascular bundle, which approximate pleural pressure, rather than by changes in alveolar pressure. The bronchovascular bundle is also the initial site of accumulation of edema fluid when animals develop pulmonary edema. where Ppa is mean pulmonary arterial pressure, Pla is left atrial pressure, and Micropuncture studies have shown that approximately half the vascular resistance in the pulmonary circulation is precapillary, and that the capillaries themselves provide a considerable portion of resistance to blood flow (Figure 46-3). Unlike the arterioles in the systemic circulation, the small arteries in the pulmonary circulation neither provide large resistance nor dampen the arterial pulsations; consequently, pulmonary capillary blood flow is pulsatile. The pulmonary veins provide little resistance to blood flow. Blood flow is preferentially distributed to the dorsocaudal region of the lung of standing quadrupeds (Figure 46-4). This distribution is accentuated by exercise and may even persist when posture changes during anesthesia. The branching pattern of pulmonary arteries and arterioles and the relative resistances of each vessel are the major determinants of blood flow distribution. The overall effects of lung volume on PVR reflect opposing effects on alveolar and extra-alveolar vessels (Figure 46-5). At residual volume, PVR is high because extra-alveolar vessels are narrowed. As the lung inflates to functional residual capacity, resistance decreases, primarily because of dilation of extra-alveolar vessels (arteries and veins). Further inflation above functional residual capacity increases PVR, primarily because alveolar capillaries are flattened by the high tension in the stretched alveolar septa. Capillaries become progressively more elliptical and therefore offer more resistance to flow.
Pulmonary Blood Flow
Pulmonary Circulation
Functionally, Pulmonary Blood Vessels Can Be Classified as Alveolar and Extra-Alveolar Vessels
The Pulmonary Blood Vessels Offer a Low Resistance to Flow
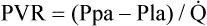
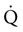 is cardiac output.
is cardiac output.
The Distribution of Pulmonary Blood Flow Within the Lung Is Influenced by Several Factors
Passive Changes in Vascular Resistance Result from Changes in Vascular Transmural Pressure
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pulmonary Blood Flow
Only gold members can continue reading. Log In or Register to continue