Fig. 1.
(a) Mean ± SEM rates of agonistic behavior for animals categorized as dominant (ranked 1 and 2) and subordinate (ranks 3 thru 5). Dominant females receive less aggressive behavior (closed square) than those categorized as subordinate, while subordinate animals emit more submissive behaviors (open square) than dominant animals. (b) Mean ± SEM change of serum cortisol at 0800 and 1100 hours following dexamethasone administration at 1750 hours the evening before for dominant and subordinate females. Asterisk denotes significantly greater decrease in cortisol in dominant compared to subordinate females. (c) Mean ± SEM change in serum cortisol 15 and 30 min following ACTH administration for dominant and subordinate females. Asterisks denote significant status differences in increases of cortisol levels. Reproduced from (53).
Subordinate status also results in reduced control over an individual’s social and physical environment (54). This exposure to daily stressors leads to long-lasting physiological alterations in the functioning of the LHPA axis that are similar to those associated with stress-induced disorders in humans. Subordinate macaques have enlarged adrenal glands (55), show diminished glucocorticoid negative feedback as assessed by a dexamethasone suppression test (Fig. 1b), and exhibit diminished cortisol reactivity in response to ACTH administration (Fig. 1c) (56). Thus, the psychogenic, uncontrollable, unpredictable nature of the imposition of social rank in macaque social groups leads to a dysregulation of the LHPA axis, providing a unique model to study how chronic psychosocial stress and dysregulation of the LHPA axis affect behavior and physiology as it relates to human health (47, 50, 53, 57–63).
2.2 Social Subordination as a Model of Stress-Induced Alterations in Feeding Behavior in Women
Recent implementation of an automated feeding system that monitors continuous food intake in socially housed monkeys at the Yerkes National Primate Research Center has provided the opportunity to quantify food intake in socially housed monkeys (64) and assess how varying dietary conditions influence food intake and energy balance differentially in dominant and subordinate monkeys.
2.2.1 Availability of a Standard Laboratory Diet
When fed a standard laboratory monkey diet, low in sugar and fat and high in fiber (53, 65), subordinate females weigh significantly less than dominant animals (Fig. 2a). This decreased body weight is associated with reduced fat, but not lean, mass (Fig. 2a). Additionally, subordinate females have lower levels of circulating leptin and insulin, and higher levels of adiponectin compared to dominant females (65). The reduced body weight in subordinate females corroborates findings of diminished body weight in rodents exposed to repeated restraint stress (66), chronic variable stress (67), or exposure to a predator (68). These results in rodents are consistent with other data from rodents showing that exposure to chronic stressors (69, 70) or CRH administration (71–74) attenuates intake of these low calorie, laboratory diets.
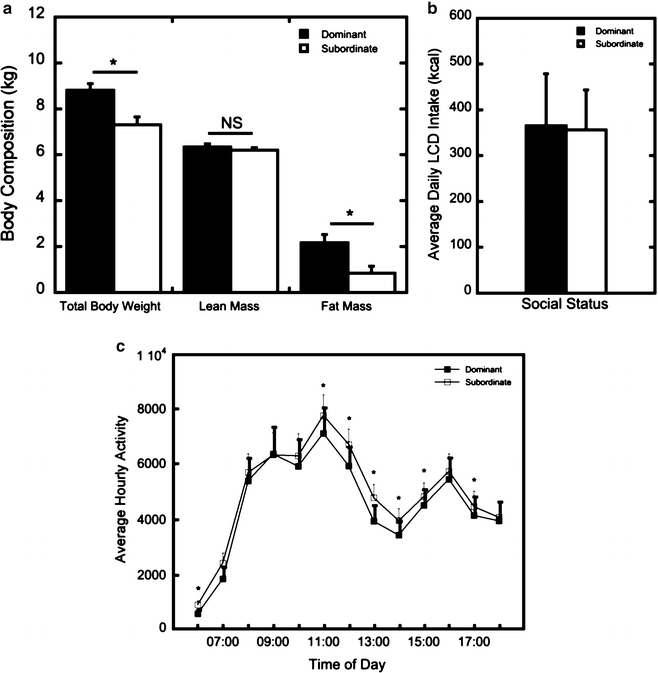

Fig. 2.
(a) Body weight and body composition (kg) in dominant (dark bar ) and subordinate (open bar ) females. Asterisks denote increased body weight due to increased fat mass in dominant compared to subordinate females. (b) Average daily caloric intake (kcal) of a standard low calorie diet (LCD) in dominant (dark bar ) and subordinate (open bar ) animals. (c) Average daily daytime activity in dominant (dark square) and subordinate (open square) females. Asterisks denote increased activity in subordinate animals compared to dominant females.
The lower body weight and fat mass in subordinate animals could be due to lower intake of the LCD, greater energy expenditure, or both. Assessment of LCD intake over the course of 2 weeks in socially housed animals using the automated feeders yields no status differences in LCD intake. Subordinate females do not consume fewer calories of a LCD than dominant females in this dietary environment (Fig. 2b), suggesting that the status difference in body weight is due to greater energy expenditure in subordinate females compared to their dominant counterparts. Indeed, assessment of activity bouts with Actical accelerometers, as a surrogate measure of energy expenditure (75), in these group-housed animals, shows that subordinate animals are more active during daytime hours, suggesting that subordinates expend more overall energy over the course of a day than do dominant animals (Fig. 2c). Increased activity in subordinate animals could indeed be a consequence of the constant threat of aggression and harassment and the need to avoid and withdraw from higher ranking females. Nonetheless, these data indicate that lower body weights in subordinate females are associated with higher levels of activity.
The imposition of social subordination following new group formation (47) reduces body weight, and these lower body weights are sustained over time (76). It is important to emphasize that these observations occur in the presence of a standard LCD. The more relevant question as it pertains to the increase in emotional eating and obesity in humans is what happens to food intake and preference when the dietary environment is expanded to include highly palatable, high-calorie foods. Do socially subordinate females show a preference for a high caloric diet that is high in fat and sugar (HFSD)? And do they increase overall calorie intake in a rich dietary environment when a choice of food is available, analogous to the dietary environment in human societies?
2.2.2 Availability of a Diet Choice Including Highly Palatable Food
Availability of a HFSD in combination with a LCD for just 2 weeks has a profound impact on caloric intake, most notably for subordinate females. Although both dominant and subordinate females prefer to consume the HFSD when given a choice, overall caloric intake is markedly different in dominant and subordinate animals during this diet condition. While dominant females consume similar amounts of total calories during both the choice condition and LCD-only conditions, subordinate females significantly increase total caloric intake when HFSD is offered concurrently with a LCD option. This augmented intake of calories upon HFSD availability in subordinate animals is greater than both subordinate baseline intake during the LCD-only condition and dominant female intake under the choice condition (Fig. 3). These data in monkeys corroborate findings from a number of rodent models (77–79) and data from humans (3, 5, 6), indicating that exposure to stress facilitates augmented calorie intake and preference for a high-calorie diet.
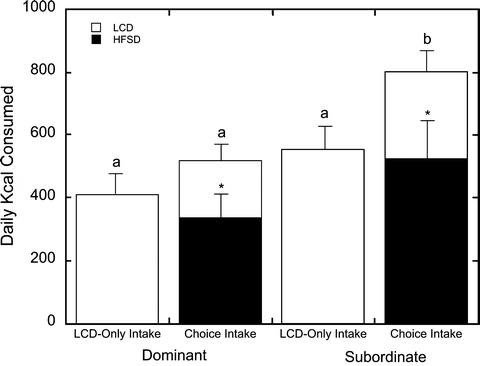

Fig. 3.
Average daily caloric intake (kcal) in dominant and subordinate females during a no choice and choice diet condition. Open bars represent intake of the low calorie diet (LCD) and closed bars indicate caloric intake of the high-fat and -sugar diet (HFSD) diet. Asterisks denote overall preference of the HFSD over the LCD during the choice condition. Letters denote differences in overall caloric intake during each diet condition. Subordinate animals with a choice to consume a HFSD increase their total caloric intake to levels higher than those seen in subordinates on a LCD diet only and to dominant females, regardless of diet condition. Reproduced from (56).
Despite having only 2 weeks of access to this rich dietary environment, the increase in caloric intake during a choice diet condition is associated with an increase in body weight in subordinate females (45). Because the data indicate that appetite in dominant animals is unaffected by dietary environment, it is plausible that the efficacy of satiety signals could be diminished and orexigenic signals augmented in subordinates (45), resulting in increased caloric intake among subordinate females during the choice diet condition. Taken together, these data indicate that the chronic psychosocial stress experienced by subordinate female monkeys increases total caloric intake only when these females are exposed to a rich dietary environment. These findings are directly relevant to the increase in emotional feeding and obesity in humans that occurs when the dietary environment includes highly palatable, high-calorie foods (21, 23).
2.3 Long-Lasting Effects of HFSD Availability on Subsequent LCD Food Intake
One question that is also of interest as it pertains to emotional eating and obesity in humans is why attempts to lose weight often fail (7). To assess how previous exposure to diets high in fat and sugar might affect food intake in a “healthy” dietary environment, similar to what humans strive for when “dieting,” caloric intake of a LCD upon the removal the HFSD was quantified (56). As illustrated in Fig. 4a, dominant monkeys continued to eat a similar number of calories regardless of diet history. In contrast, subordinate females continued to eat significantly more calories compared with dominant females during this post HFSD phase (Fig. 4b). While caloric intake was lower than observed during the choice phase when HFSD was available, it was significantly higher than the previous LCD phase before any exposure to a HFSD (Fig. 4b). These data suggest that a background of chronic, psychosocial stress can interact with diet history (and exposure to a HFSD) to increase caloric intake even in a healthy dietary environment (56).
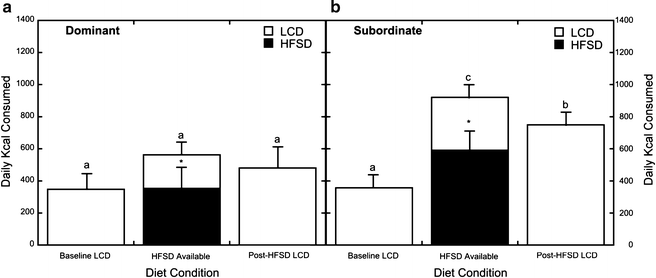

Fig. 4.
Average daily caloric intake in dominant (a) and subordinate (b) females. Open bars represent intake of the low calorie diet (LCD) and closed bars indicate caloric intake of the high-fat and -sugar diet (HFSD). Asterisks denote overall preference of the HFSD over the LCD during the choice condition. Letters denote differences in intake during each diet condition. Subordinate animals consume more overall calories of the LCD diet following exposure to a HFSD than they did prior to HFSD exposure (b), whereas prior HFSD exposure does not affect caloric intake of a LCD in dominant animals following removal of HFSD. Reproduced from (56).
The lasting effects of HFSD exposure on appetite regulation in subordinate female macaques could be due to changes in specific satiety signals. While intake of a HFSD is also associated with an increase in insulin and glucose levels in all animals regardless of social status, only subordinate females continue to consume more overall calories once the HFSD is removed (56). The finding that leptin levels are increased only in subordinate females following HFSD exposure during the subsequent period of increased LCD-only intake (56) further supports the notion that satiety signaling might be altered in subordinate females (56). Studies in rodents have shown that glucocorticoids can lead to both weight gain and increased feeding by inducing leptin insensitivity (80, 81). Additionally, excess glucocorticoids counteract the activity of insulin and can facilitate the development of insulin insensitivity, resulting in increased lipogenesis, central adiposity, and leptin levels (82). A consequence of these physiological changes include augmented food intake and increased body weight as described in individuals suffering from excess cortisol levels due to Cushing’s disease (83).
The diet interventions we have used in our rhesus monkey model were tested for 2–3 week durations, too brief to increase fat mass. However, longer HFSD exposure can lead to insulin resistance and reduce satiety signaling in subordinate females (84), which could possibly contribute to excess calorie consumption. Taken together, these data suggest that the exposure to a HFSD alters sensitivity to signals that are critical for the maintenance of energy homeostasis and could explain the high failure rate of weight loss attempts among human populations.
3 Notes
3.1 Individual Variability in Food Intake
While social status and stress exposure account for a significant proportion of variance in total caloric intake when a HFSD is available, there is nonetheless variability in feeding behavior within each social status category in this dietary environment. Animals classified as dominant show some variability in HFSD intake during its availability (Fig. 5), whereas females categorized as subordinate show a greater amount of variance in HFSD intake. This variability among members of each social status category indicates that other variables interact with social status to contribute to this feeding phenotype. Genetic factors confer individual differences and contribute to increased individual vulnerability to stress-induced disorders (85). An example of this is the polymorphic region in the promoter of the serotonin reuptake transporter, whose short allele variant has been linked to increased susceptibility to depression (85). Likewise, a polymorphism in the dopamine D2 receptor gene is linked to increased incidence of obesity (86). Thus, polymorphisms in genes regulating feeding behavior and stress axis reactivity could interact with the environment and account for the variability observed in emotional feeding within subordinate monkeys (87–89). Furthermore, epigenetic changes in gene expression due to life experiences and the environment likely facilitate individual variability in emotional feeding and other stress-induced phenotypes (90). Importantly, social subordination in female rhesus monkeys provides us with a unique model to differentiate how these factors contribute to the feeding phenotypes observed in both dominant and subordinate animals.
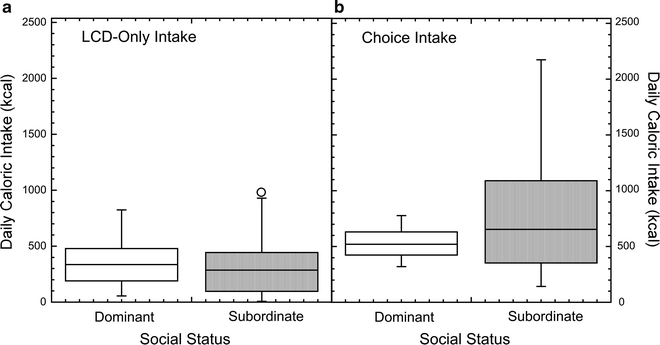

Fig. 5.
Box plots depicting variability of overall caloric intake and emotional feeding within animals of each social status categorization during (a) the LCD-only with no previous history of HFSD and (b) a diet choice condition where HFSD was available.
3.2 What Sustains Emotional Feeding?
The mechanisms responsible for the increased motivation to consume calories in the presence of a highly palatable diet in individuals experiencing chronic stress remain uncertain. A possible explanation for altered feeding behavior is altered sensitivity to appetite and satiety signals under conditions of chronic stress. An example of this is the increased sensitivity to ghrelin observed in subordinate females, and not dominant females, that is linked to increased caloric intake and LHPA dysregulation in subordinate animals (91). Leptin signaling might also be disrupted by social subordination as HFSD intake in subordinate females increases circulating leptin levels while still maintaining increased caloric intake (56). Thus, changes in sensitivity to appetite and satiety signals can occur in concert with one another and depend on the dietary environment. Future studies are necessary to delineate how psychosocial stress exposure and activity of the LHPA axis influence the regulation of orexigenic and satiety signals in complex dietary environments to dysregulate feeding behavior.
Another possible mechanism is that comfort food ingestion diminishes activation of the stress response, as has been shown in rodents (22, 78, 92, 93) and in high-stress premenopausal women (94). However, because glucocorticoids are a critical component in initiating emotional feeding (95–97), a reduction in stress hormone responsivity by comfort foods is likely not a sustaining factor that maintains this phenotype. In contrast, the availability of a HFSD in female macaques increases the diurnal rhythm of cortisol (45) and HFSD consumption augments cortisol responsivity to an acute social separation stressor, regardless of social status (45, 56). These data in subordinate female monkeys not only support data from rodents with access to a HFSD (98–102), but also are consistent with clinical data linking enhanced LHPA activity to measures of central adiposity (103–105). This increased LHPA activity linked to ingestion of HFSD in subordinate monkeys supports the notion that emotional feeding is a behavior that results in the reinforcement and continued motivation to engage in emotional feeding through an increase in glucocorticoids and LHPA activation.
Because ingestion of HFSDs is linked to the activity of the LHPA axis and LHPA axis activity can modulate behavior (53, 91), it could be possible that consumption of these highly palatable diets might affect mood and socioemotional behavior. While some studies in rodents and in rhesus monkeys have shown that availability of a HFSD reduces aggression and anxiety-like behavior (45, 92, 101, 106, 107), other data from monkeys show that HFSD availability has no effect on socioemotional behavior (56). Additionally, while the lack of behavioral effects upon HFSD availability could indicate that HFSD intake does not affect behaviors, it is more plausible that the increased LHPA activation associated with HFSD availability is actually having an adverse or neutral effect on socioemotional behaviors in this particular social context. Indeed, if comfort food ingestion actually increases stress hormone responsivity, consumption of these diets may actually be anxiogenic. Further studies are necessary to determine how HFSD availability and ingestion affect social and emotional behaviors in this model and other social contexts. Additionally, determining how diet affects behavioral responses to acute threatening situations is important to better understand why individuals engage in emotional eating.
A final hypothesis for sustained emotional feeding involves the reward pathways. Chronic psychosocial stress exposure in humans increases individual vulnerability for addictive phenotypes (108), including psychostimulant abuse (109), by reducing dopamine D2 receptors in mesolimbic regions and producing a hypodopaminergic condition in corticolimbic regions of the brain that are important for reward processing (110–112). Intake of calorically dense diets reduces dopamine D2 receptors in these reward regions of the brain in obese humans (113, 114) and in some animals (113). Additionally, studies in rats and in humans indicate that HFSD availability activates reward circuitry in forebrain structures (115–117). Because social subordination in macaques also results in a reduction in dopamine D2 receptor binding potential (61, 118), it is likely that emotional eating in subordinate animals may act as a self-prescribed treatment for increasing the activation of an already dysfunctional reward system (56). Furthermore, the long-lasting effects of HFSD exposure on caloric intake among subordinate monkeys in a healthy dietary environment could be based on a continuing drive to engage a hypoactive dopaminergic system in the absence of a HFSD (56).
4 Conclusion
The ongoing studies of food intake in socially subordinate female rhesus monkeys indicate that social subordination provides an important model to study stress-induced emotional eating and its impact on obesity in females. The use of this translational model in nonhuman primates may help fill gaps in knowledge surrounding stress-induced alterations in feeding behavior and the interaction with dietary environments for women. Critical questions regarding emotional eating in females that remain unanswered include, but are not limited to: What is the underlying neurobiological mechanism that initiates and sustains emotional feeding? Does hyperphagia in a healthy dietary environment persist indefinitely following HFSD exposure, and if so, what are the mechanisms that maintain this feeding phenotype? Do satiety signals become ineffective at curbing emotional feeding? How does HFSD exposure alter LHPA activity and how do these changes affect socioemotional behavior? How does long-term (greater than 2 weeks) availability of HFSDs affect LHPA activity, metabolism, and food intake superimposed on a background of social chronic stress? What accounts for individual variability in stress-induced changes in feeding and socioemotional behavior and physiology? Are there behavioral interventions or pharmacotherapies that might rescue or curtail emotional feeding in vulnerable individuals?
The animal model of stress-induced emotional eating described in this chapter will allow us to answer these questions using tools such as genetics, epigenetics, and neuroimaging simultaneously. These results will be critical for understanding the etiology of emotional eating and factors that might increase individual vulnerability to stress-induced eating and obesity, thus providing the basis for treatments that may benefit millions of individuals worldwide (Fig. 6).

Fig. 6.
Cartoon of stress-induced emotional feeding in female monkeys.
Acknowledgments
We would like to thank Jennifer Whitley, Shannon Bounar, Jodi Godfrey, Christine Marsteller, Jonathon Lowe, Rebecca Herman, Robert Johnston, and Gregory Henry for their expert technical assistance in conducting the feeding studies. We also thank Dr. Donna Toufexis for helping shape our understanding of stress–feeding relationships. These studies would not have been possible without the dedication of the animal husbandry staff at the Yerkes National Primate Research Center (YNPRC) and support by NIH grants HD46501 (MW), MH081816 (DT), and RR00165, and F31MH085445 (VM). Further support was provided by the Center for Behavioral Neuroscience through the STC Program of the National Science Foundation IBN-9876754. The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
References
1.
World Health Organization (2010) Vital signs: state-specific obesity prevalence among adults United States, 2009. MMWR Morb Mortal Wkly Rep 59:951–955
2.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


