Fig. 1.
The stressful procedure: rats were prevented from accessing HPF, but were able to see and smell it.
In this 15 min period, rats engaged in repeated movements of the forepaws, head, and trunk aimed at obtaining the HPF without being able to reach it. Rats underwent the stressful procedure between 1000 and 1200 hours. After 15 min, the cup was placed inside the cage, so that HPF became accessible to the stressed rats.
As shown in Fig. 2, exposure to HPF without access to it, increased corticosterone (CORT) levels in serum samples obtained from rats sacrificed 15 min after the beginning of the stressful procedure, both in rats submitted to food restriction (R + S) and in rats not submitted to food restriction (NR + S). These findings provide evidence that the procedure was indeed able to evoke a stressful response in the animals. This response was rather short lasting, since 60 min after removal of the HPF cups from the wall of the cage (without allowing the rats to access HPF), CORT levels returned to control (NR + NS) levels.
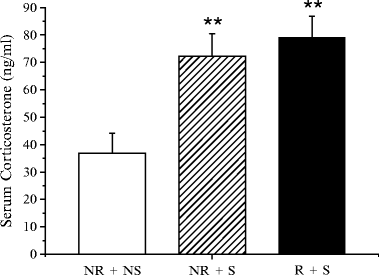

Fig. 2.
CORT levels in control rats (NR + NS ) and in rats exposed to stress with (R + S ) or without (NR + S ) cycles of food restrictions. In R + S and NR + S rats CORT levels were measured 15 min after the beginning of the stressful procedure. The values shown are the mean ± SEM. Statistical differences from controls (NR + NS ): **p < 0.01.
This type of stress offers several advantages over the electric foot-shock stress. First, it is a mild stress that, unlike the electric foot-shock, never induces fear and freezing, but elicits a robust behavioral activation. Second, the stressful experience implies a relationship with HPF that resembles the approach-avoidance stress over “forbidden” food that is very common among human binge eaters, thus providing a further element of face validity.
2.2 The HPF
Rather that using sweet biscuits, which usually generate a large amount of spillage, a HPF formulated as a paste was employed. It was obtained by mixing (a) Nutella (Ferrero, Alba, Torino, Italy) chocolate cream (5.33 kcal/g; 56%, 31%, and 7% from carbohydrate, fat, and protein, respectively), (b) grounded food pellets 4RF18 (Mucedola, Settimo Milanese, Italy), and (c) water in the following percent ratio: 52% Nutella, 33% food pellets, 15% water.
Rats exhibited a pronounced intake of HPF, since the first exposure to it on day 5 of the first cycle (about 5.5 g per rat); the intake increased on the following day (about 9 g per rat). On days 13 and 14 of the second cycle HPF intake did not increase further.
2.3 The Experimental Procedure
Before tests, female rats were divided into four weight-matched experimental groups (usually N = 9 per group):
Group 1: nonrestricted and not exposed to stress (NR + NS)
Group 2: restricted and not exposed to stress (R + NS)
Group 3: nonrestricted and exposed to stress (NR + S)
Group 4: restricted and exposed to stress (R + S)
Rats were maintained on 3 consecutive 8-day cycles, followed by the final test on day 25, as follows:
Rats in Group 1 (NR + NS), the control group, had ad libitum access to chow for 4 days, on days 5–6 they received chow ad libitum + HPF for 2 h; on days 7–8 they had chow ad libitum; on day 25 they were not exposed to stress.
Rats in Group 2 (R + NS) had chow restricted to 66% of the normal intake for 4 days; they were offered chow ad libitum and HPF for 2 h on days 5–6, and only chow on days 7–8; on day 25 they were not exposed to stress. Restriction to 66% of normal chow intake was defined for the first cycle on the basis of the intake measured in the 6 days before the beginning of the experiment. For the second and third cycle, 66% restriction was defined on the basis of the intake of nonrestricted rats during the last 2 days of the cycles, in which rats received only normal chow ad libitum.
Rats in Group 3 (NR + S) had chow and HPF as controls, but were exposed to stress on the test day (day 25).
Rats in Group 4 (R + S) had food available similar to Group 2, but were exposed to stress on day 25.
The 8-day cycle was repeated three times, but in the third cycle the animals did not have access to HPF on days 21 and 22. On day 25, free access to HPF and chow was offered and the intake was measured in the first 2 h, taking care to collect any spillage. A minimum of three caloric-restriction and refeeding cycles were run, as in previous studies (31–33).
Body weights and food intake were recorded daily. Food intake was expressed as the mean kcal/kg ingested ± S.E.M. By the last day of refeeding, body weight and food intake of restricted rats were not statistically different from those of nonrestricted rats (Fig. 3), thus eliminating the potentially confounding influence of hunger or energy deficit.
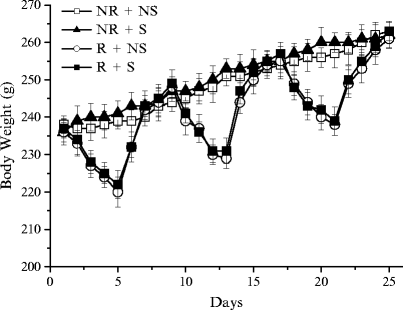

Fig. 3.
Effect of the three restriction/refeeding cycles on body weight (g) of the four groups of female rats. The values are the mean ± SEM.
3 Anticipated Results and Notes
3.1 Anticipated Results
The experiments were initially carried out in four different groups of rats; the overall ANOVA revealed a statistically significant difference in 2-h HPF intake in the different groups of rats in all of the experiments.
As shown in Fig. 4, HPF intake in the R + S group was markedly higher than in the control (NR + NS) group at the different times of observation. The intake of HPF by R + S rats began immediately after access to it; these animals never engaged in competing behaviors, but continuously remained over the cup containing HPF and focused their attention on its intake. HPF intake was very pronounced in the first 15 min of access to it. After the first 15 min, the additional intake of the four groups was similar, thus at 2 h the cumulative intake of the R + S group remained higher than that of the other groups. HPF intake of the groups NR + S or R + NS were neither significantly different from that of controls. As far as the intake of food pellets is concerned, the ANOVA revealed no significant difference among groups. The intake of food pellets was affected neither by food restriction, stress, nor the combination of both.
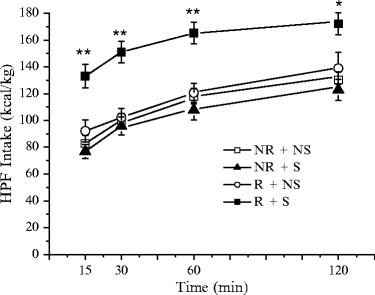

Fig. 4.
HPF intake at different times after access to it on the test day (day 25). The values shown are the mean ± SEM. Statistical differences from controls (NR + NS ): *p < 0.05; **p < 0.01; where not indicated, the difference was not statistically significant.
The rats’ body weights were markedly reduced during the 4 days of food restriction, but rapidly recovered to levels of controls by the end of each cycle. On the test day, the body weights of the four groups of animals, as well as their food intake in the previous 24 h, were not statistically different.
Although the ANOVA gave a statistically significant effect in all the experiments carried out with this model, a considerable interanimal variability was evident: while in some animals the increase in HPF was very pronounced, almost doubling the intake of NR + NS rats, a small percentage of rats had a HPF intake strictly similar to that of NR + NS animals.
3.2 Influence of the Ovarian Cycle in This Model of BE
Searching for the reasons accounting for the observed variability, we thought it would be interesting to evaluate whether it might be related to the ovarian cycle of the female rats. Indeed, menstrual cycle and gonadal hormones are known to influence eating behavior in healthy females. Reductions in food intake and meal size occur in the peri-ovulatory phase, following the rise of estradiol secretion, while food intake generally increases in the luteal phase, when plasma progesterone levels are high (44–47).
A large body of evidence shows a significant association between changes in ovarian hormone levels and BE episodes during the menstrual cycle in women with BN. For instance, several studies have found an increased number of BE episodes in the premenstrual compared to the menstrual period (48–50). Moreover, a relatively recent longitudinal study reported that the increase in estradiol and decrease in progesterone prospectively predict reduction of binge frequency (51). This negative association has been replicated by Klump et al. (52) in two community samples of women, in which decreases in estrogens were associated with overeating and greater likelihood of feeling “out of control.”
The ovarian hormone estradiol is involved in the physiological control of feeding via interactions with anorexigenic peptides like cholecystokinin (53–58), insulin, and leptin (59), as well as with orexigenic peptides like neuropeptide Y (60–62), ghrelin (63–65), and melanin-concentrating hormone (66, 67). Estradiol also influences serotonin release and degradation, as well as activation of its receptors (68). Estrogens modulate dopaminergic systems, and this modulation may involve environmental estrogen exposure (69).
In female rats, the estrous cycle is usually 4 days in length, with four distinct phases (70):
1.
Proestrus (P): estradiol rises to the highest levels and progesterone levels are low at the beginning and rapidly rise and descend toward the end.
2.
Estrus (E): estradiol and progesterone levels rapidly decline.
3.
Metestrus (D1): estradiol levels are low and progesterone levels begin to rise.
4.
Diestrus (D2): estradiol levels are rising and progesterone levels decline.
During the estrus phase rats, like other animals, show a transient decrease in food intake while they eat most during the diestrus phase (71, 72). Most physiological estradiol effects have a latency of 12 h or more, ∼30 h in the study by Asarian and Geary (73), since the activation of estrogen receptors stimulates transcription factors. Therefore, decreases in food intake during the estrus phase may be caused by the preceding increase in estradiol secretion in the proestrus phase.
Bilateral ovariectomy (OVX) produces a rapid increase in food intake, body weight, and adiposity (74, 75), removing estrogen’s inhibitory effect. These responses to OVX can be normalized by a regimen of estradiol treatment with a single subcutaneous injection of estradiol (76) that produces hormonal changes in plasma similar to those observed in cycling rats. Estrogens regulate body adiposity and fat distribution through the ER alpha (ERα) and beta (ERβ) receptors (77, 78). However, only ERα has been proposed to have a major influence on energy homeostasis (79). The effects of estradiol on food intake appear to be mediated by ER within the hypothalamus, particularly ventromedial and paraventricular nucleus (PVN) (54, 65, 80).
Little is known about the role of estrogens on BE in rats. Yu et al. (81) have shown that estradiol reduces binge size in female rats, in which a highly restricted schedule of access to fat led to binge-like intake of fat in a 1-h test. However, this effect was evident at the start of the study but not at the end, when bingeing was fully established.
In our study, the ovarian cycle was monitored by examination of vaginal smears obtained with a moistened cotton swab and warm physiological saline; afterwards, the samples were transferred onto glass slides for microscopic analysis. Following examination of vaginal smears on the test day, immediately after the HPF intake test, statistical analysis revealed that HPF intake was significantly lower during the estrus phase both in NR + NS and R + S rats (82). HPF intake of R + S rats was significantly higher than that of NR + NS rats during proestrus, metaestrus, and diestrus; however, during estrus it was only slightly higher in R + S rats, and the difference between the two groups was not statistically significant (Fig. 5). These findings indicate that BE in our model does not occur during the estrus phase and that the observed variability in the BE response can be almost completely abolished if female rats in estrus are not included in the statistical evaluation. These findings encourage further investigations of the mechanisms by which ovarian hormones, particularly estradiol, control BE.
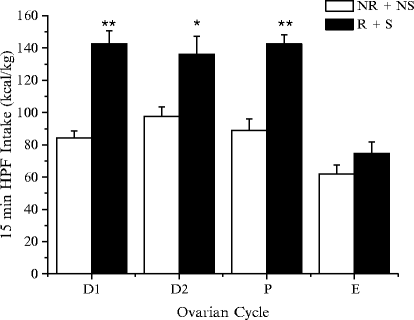

Fig. 5.
HPF intake on the test day (day 25) in R + S and in NR + NS female rats in the different phases of the ovarian cycle: D1 (Metestrus), D2 (Diestrus), P (Proestrus), E (Estrus). The values shown are the mean ± SEM. Statistical differences from controls (NR + NS ): *p < 0.05, **p < 0.01; where not indicated, the difference was not statistically significant.
3.3 Drugs so Far Tested in This Model
3.3.1 Drugs to Evaluate the Predictive Validity of the Model
To test the predictive validity of this preclinical model of BE, the following three drugs have been tested: sibutramine, fluoxetine, and topiramate. They were chosen because clinical studies have reported that they may be effective in the treatment of bingeing-related eating disorders (12–17, 83–88). Sibutramine (Reductil®) is a centrally acting serotonin–noradrenaline reuptake inhibitor, which has been reported to reduce food intake and to increase energy expenditure (89). Fluoxetine (Prozac®) is a selective serotonin reuptake inhibitor; it reduces food intake by affecting appetite and satiety. In addition, fluoxetine appears to have positive effects on the control of impulsive and compulsive symptoms associated with psychiatric conditions like obsessive–compulsive disorder, BN, and hypochondriasis (86). Topiramate (Topamax®) was developed as an antiepileptic drug; however, clinical trials have shown that it inhibits BE (12, 13). Sibutramine reduced HPF intake not only in the R + S, but also in the other experimental groups, suggesting that it exerts a rather general inhibitory effect on food consumption. Fluoxetine, at the intragastric dose of 3 mg/kg, significantly reduced HPF intake only in the R + S group. At the dose of 10 mg/kg, the effect of fluoxetine was not selective and it significantly reduced HPF intake in all four groups. On the other hand, topiramate, even at the highest dose (60 mg/kg) given by gavage, selectively reduced HPF intake only in R + S rats.
These findings, particularly those obtained with topiramate, suggest that the preclinical model described by Cifani et al. (39) exhibits interesting elements of predictive validity. In addition, further elements of face validity for this model derive from the finding that the occurrence of BE in the female rats employed is influenced by the ovarian cycle, as it happens in women.
3.3.2 Drugs Targeting Stress Mechanisms
As stated above, a large body of evidence suggests that stress may represent a key determinant of BE in patients suffering from BED or BN (21, 22). The important role of stress in the etiology of BE is emphasized by the finding that obese individuals with BED exhibit activation of the hypothalamic–pituitary–adrenal (HPA) axis, and their cortisol levels are higher in comparison with those of obese individuals without BED (90, 91). Moreover, salivary cortisol levels are positively correlated with BE severity (92), and higher blood cortisol levels in response to stress predict greater intake of sweets (93). Therefore, we thought it would be interesting to evaluate the effect of pharmacological agents directed at stress mechanisms in our BE model.
Corticotropin-releasing factor (CRF) is a 41 amino acid polypeptide that represents the major mediator of the stress response at both hypothalamic and extrahypotalamic levels. Hypothalamic CRF, which is synthesized in PVN neurons, controls the production and release of adrenocorticotropic hormone (ACTH) from pituitary corticotropes through activation of CRF-1 receptors. In turn, ACTH released into the blood stream stimulates glucocorticoid synthesis and secretion from the adrenal cortex (94, 95). The brain extrahypothalamic CRF systems (including amygdala, extended amygdala, and medial septum) appear to be involved in emotional and motivation processes, and CRF-1 receptors are the main mediators of these effects (95). CRF-1 receptor antagonists have been reported to inhibit negative emotional states in drug dependence, and to block stress-induced reinstatement of drug seeking for cocaine, opiates, ethanol, and nicotine (40, 96–102). Apparently, the bed nucleus of the stria terminalis, the median raphe and the ventral tegmental area are important sites for the effect of CRF-1 receptor antagonists on stress-induced relapse in drug abuse (103–106). Interestingly, CRF-1 receptor antagonists have been reported to reduce stress-induced palatable food seeking in rats (101, 107), and to reduce withdrawal symptoms in conditions of intermittent access to palatable food (108).
In addition to CRF, glucocorticoids have received great attention in relation to motivational responses to stress. Increased CORT levels represent a hormonal marker of BE in rats in the cyclic food restrictions + stress models (33, 39, 109). Palatable food intake has been shown to blunt activation of the HPA axis (110–112), suggesting that it may be used, at least in part, as self-medication from stress. It is noteworthy that CORT levels are also increased following high-fat diet removal (113), as they are during withdrawal from addictive drugs (112, 114). CORT appears to be implicated in the motivation to seek rewarding substances (107, 115–119), probably by evoking dopamine release in the nucleus accumbens (120, 121).
3.4 Examined Components of the Stress Response
3.4.1 CRF-1 Receptor Antagonist
In the experiments carried out by our group, and published as an abstract at the 2011 Meeting of the Society for the Study of Ingestive Behavior (122), the role of CRF on BE was investigated by means of the highly selective CRF-1 receptor antagonist, R121919 (123–125), provided by Dr. Kenner Rice, NIDA/NIH, Bethesda (USA).
As shown in Fig. 6, the subcutaneous administration of this CRF-1 receptor antagonist significantly reduced the increase in HPF intake of the R + S group at the dose of 10 mg/kg and completely suppressed it at 20 mg/kg. No effect was observed at 5 mg/kg. While suppressing the increase in HPF intake in the R + S group, doses of 10 or 20 mg/kg of R121919 did not significantly reduce HPF in the control group (NR + NS). Thus, the effect of R121919 is clearly selective, and it is not producing a general inhibition of food intake, like that observed for serotonergic drugs, such as fluoxetine or sibutramine (39).
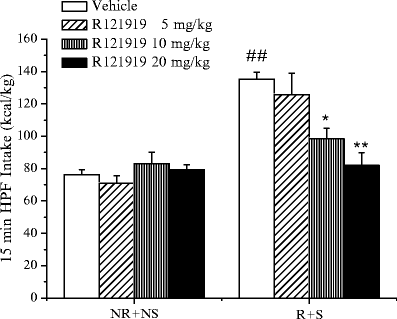

Fig. 6.
The effect of subcutaneous administration of the CRF-1 receptor antagonist R121919 on HPF intake on the test day in R + S and NR + NS rats. The data are the mean ± SEM. Statistical differences: ## p < 0.01 vs. NR + NS vehicle; *p < 0.05 and **p < 0.01 vs. R + S vehicle.
3.4.2 CORT
As already reported (39), the stressful procedure adopted in our model induces a marked increase in serum CORT levels. CORT has been implicated in the motivation to seek rewarding substances (107, 115–119), raising the question whether it might be involved in the BE response. To address this issue, stress exposure was substituted with an intraperitoneal injection of CORT in rats already submitted to cycles of food restriction/refeeding. If CORT mediates the effect of stress to evoke BE, R + NS rats should express BE following treatment with CORT. Contrary to this prediction R + NS rats, exposed to a wide range of CORT doses, failed to show an increase in HPF intake in comparison to NR + NS rats (122).
3.4.3 Metyrapone
Metyrapone is CORT synthesis inhibitor. When it was administered by intraperitoneal injection at different doses, it failed to prevent BE in R + S rats and did not modify HPF intake in NR + NS rats (122).
Taken together, these findings do not support a direct role of CORT in the etiology of BE. Studies concerning drug abuse have shown that high circulating levels of glucocorticoids can “sensitize” the CRF systems in extrahypothalamic sites that are involved in behavioral responses to stressors (126–130). The finding that BE is observed only after three cycles of food restriction/refeeding might imply the occurrence of processes of brain plasticity/sensitization; activation of the HPA axis may lead to subsequent sensitization of extrahypothalamic stress mechanisms, as described for drug addiction (131–133).
Thus, the effect of CRF on BE is probably exerted not through activation of the HPA axis in the hypothalamus, but in extrahypothalamic sites involved in emotional and motivational control. Many of these effects of CRF have been localized in the extended amygdala, a macrostructure composed of basal forebrain structures, including the central medial amygdala, the bed nucleus of the stria terminalis, and a transition zone in the posterior part of the medial nucleus accumbens, the posterior shell (134). Key elements of the extended amygdala include not only neurotransmitters associated with the positive reinforcing effects of drugs of abuse, but also major components of the brain’s stress systems associated with the negative reinforcement of dependence (132).
Several pieces of evidence suggest similarities between drug addiction and compulsive eating disorder. For example, CORT is increased during high-fat diet removal (113), as it is during withdrawal from addictive drugs (114). This may set up a vicious addiction-like cycle of eating palatable food when stressed, then suffering the consequences of palatable food withdrawal, a stressor in itself (108). Cottone et al. (108) found that rats with intermittent access to palatable food elicit symptoms of withdrawal when the palatable food is not available, symptoms reversed by CRF-1 receptor antagonists. Thus, the results of this study provide evidence that CRF-1 receptors are involved in the control of BE and CRF-1 receptor antagonists may represent interesting tools for the pharmacotherapy of bingeing-related eating disorders.
3.4.4 Rhodiola rosea Dry Extracts
Another study of our group was aimed at investigating the effect of a dry extract of Rhodiola rosea and of its active principles in the same model of BE in female rats. R. rosea is a plant commonly used in traditional medicine for its ability to increase body resistance to physical, chemical, or biological stressors. The antistress properties have been attributed to modulation of the activity of the sympathetic system, of the HPA axis, as well as to influence on molecular chaperons like Hsp70, stress-activated c-Jun N-terminal protein kinase (JNK1), Forkhead Box O transcription factor DAF-16, cortisol, and nitric oxide (135, 136). Studies evaluating the effect of R. rosea at CRF receptors are not available, but R. rosea extracts have been reported to reduce behavioral responses evoked by central CRF administration, such as CRF-induced anorexia (137).
Administration by gavage of a dry extract of R. rosea, 10 mg/kg, significantly reduced the increase in HPF intake in the R + S group, while 20 mg/kg completely abolished it (138). While suppressing the increase in HPF intake in the R + S group, 20 mg/kg of R. rosea extract did not reduce HPF intake in the control group (NR + NS), the NR + S group, or the R + NS group; moreover, it did not modify the intake of food pellets in food-sated or food-deprived rats. The same doses of R. rosea abolished the increase in serum CORT levels, suggesting that it abolishes the stress response.
While in the present study R. rosea extract inhibited BE, Mattioli and Perfumi (137) have shown that R. rosea antagonizes the anorectic effect of stress and of CRF, increasing food intake in conditions in which it was suppressed by restraint stress or by central CRF injection. Indeed, stress is well known to be responsible for both stimulation and inhibition of feeding (41, 43), apparently depending on the intensity of the stress itself. Thus, the different effects of R. rosea on feeding behavior may likely be the consequence of its primary influence on the stress mechanisms, rather than a direct effect on orexigenic or anorexigenic mechanisms. By influencing the response to stress, R. rosea extracts can either suppress stress-induced BE or stress-induced anorexia.
R. rosea roots contain a variety of biologically active compounds, including organic acids, flavonoids, tannins, and phenolic compounds. Phenylpropane and phenylethane phenolic glycosides, such as salidroside, rosavin, syringin, and triandrin, are considered the most important active principles (135). Extracts of R. rosea are usually standardized for rosavin and salidroside; therefore, the effect on BE of purified rosavin and salidroside was evaluated in the R + S group, which is the animal group that exhibited BE. As shown in Fig. 7, salidroside (administered in amounts similar to those present in 20 mg/kg of the extract) was able to significantly reduce BE. On the other hand, the effect of rosavin (again in amounts similar to those present in 20 mg/kg of the extract) induced a lower, not statistically significant reduction of BE. These findings indicate that salidroside may represent the main active principle of R. rosea in suppressing BE.
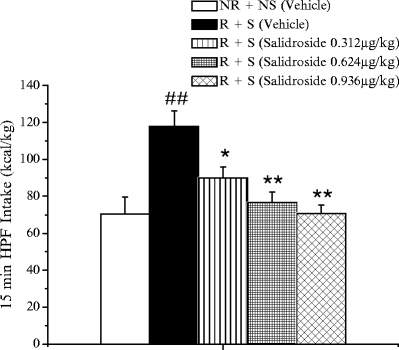

Fig. 7.
The effect of intragastric administration of salidroside or vehicle on HPF intake on the test day in R + S rats. The data are the mean ± SEM. Statistical differences: ## p < 0.01 vs. NR + NS vehicle; *p < 0.05 and **p < 0.01 vs. R + S vehicle.
3.5 Drugs Targeting Orexin Mechanisms
A large body of evidence supports a role of the orexin (OX) system in feeding behavior, particularly in the control of reward-based food intake (139). Interestingly, OX neurons in the lateral hypothalamus have been proposed to mediate reward ((95) for review); these neurons are activated by cues associated with rewards, such as food or drugs, and their activation reinstates drug seeking in rats (140–142). OX-1 receptor antagonists inhibit the hyperphagic effect of OX (143, 144) and inhibit also high-fat food self-administration (145).
Several reports have implicated OX peptides in stress responses. OX neurons in the perifornical–dorsomedial hypothalamus have been proposed to mediate stress (95, 146) through the activation of CRF-expressing neurons in the PVN of the hypothalamus and in the central nucleus of the amygdala (147). Accordingly, OX-1 receptor antagonists inhibit electric foot shock-induced reinstatement of cocaine seeking (148, 149), as well as ethanol and sucrose seeking induced by the pharmacological stressor yohimbine (149). Recent reports also support a role for OX signaling in drug reinforcement and drug abuse-induced plastic changes in the brain (150–153). Consistent with these findings, blockage of OX-1 receptors decreases ethanol, cocaine, and nicotine self-administration (141, 151, 154).
Evidence is accumulating that excessive intake of certain foods under specific conditions produces behaviors and changes in the brain that resemble an addiction-like state (155–160). Neural systems that motivate and reinforce drug abuse have been proposed to underlie behaviors associated with compulsive food seeking and food intake (161–165). These findings raise the question of whether the OX system has a role also in eating disorders characterized by compulsive binge-type episodes, such as BN and BED.
Therefore, the effects of GSK1059865, a selective OX-1 receptor antagonist, JNJ-10397049, a selective OX-2 receptor antagonist, and SB-649868, a dual OX-1/OX-2 receptor antagonist, were investigated in our experimental BE model in female rats. The results obtained, published in (166), showed that none of the OX antagonists affected feeding in NR + NS rats. However, in R + S rats SB-649868 and GSK1059865 selectively reduced HPF intake. No effect was observed for JNJ-10397049. Altogether these findings suggest the involvement of OX-1 but not OX-2 receptors in BE.
Further studies are underway to evaluate whether OX-1 receptor antagonists reduce BE by interfering with stress or reward mechanisms; however, their ability to evoke pronounced and selective suppression of BE suggests that targeting OX-1 receptor system could represent an interesting pharmacotherapeutic approach for the treatment of BE-related disorders.
4 Conclusion
At present the preclinical model of BE described in this chapter (39) has been employed by our group in at least 15 groups of female rats and, in most of these groups, the BE test has been replicated for up to five consecutive times, without evidence of development of tolerance. Following the observations related to the influence of the ovarian cycle on the BE response, and the consequent exclusion of data coming from female rats in the estrus phase of the ovarian cycle, the model proved to be highly reliable and reproducible.
The results obtained with topiramate, sibutramine, and fluoxetine indicate that the model has interesting elements of predictive validity. It exhibits evidence of face validity related to the very prompt and pronounced intake of HPF in a short period of time, which is repeated over time when animals experience the stressful procedure. Moreover, like in women, in the female rats used in this model BE is tightly linked to the hormonal fluctuations of the ovarian cycle. Lastly, the model exhibits strong construct validity, since BE is evoked by yo-yo dieting and stress, which are also considered key determinants of BE in humans.
Our BE model may be particularly suitable to investigate the role of stress mechanisms in the arousal and maintenance of the binge-type behavior. The interesting results so far obtained with the CRF-1 receptor antagonist R121919 clearly support the involvement of CRF mechanisms in BE. Further studies are needed to explore at a more systematic level the significance of CRF mechanisms not only on the expression of the BE episode, but also on the processes leading to its emergence. Studies are ongoing in our group to explore the involvement of the CRF system in the processes of brain plasticity/sensitization occurring during restriction/refeeding cycles.
We find the results obtained with the OX-1 receptor antagonists particularly interesting. The mechanisms by which OX-1 receptor antagonists suppress BE remain to be investigated. However, considering that excessive intake of certain foods produces behaviors and changes in the brain that resemble an addiction-like state (155–160), it is reasonable to speculate that OX mechanisms may be common to both drug abuse and compulsive eating behaviors. Targeting this system may offer a strategy to develop pharmacotherapeutic agents potentially effective for both addiction and eating disorders.
Acknowledgements
The authors wish to thank Dr. Kenner Rice for synthesizing R121919, Marino Cucculelli for his skilful technical assistance, and the Editor of the book for her kind invitation to contribute to it.
References
1.
American Psychiatric Association (2000) Diagnostic and statistic manual of mental disorders, IV-TR. American Psychiatric Association, Washington, DC
2.
4.
5.
6.
7.
Javaras KN et al (2008) Co-occurrence of binge eating disorder with psychiatric and medical disorders. J Clin Psychiatry 69:266–273PubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


