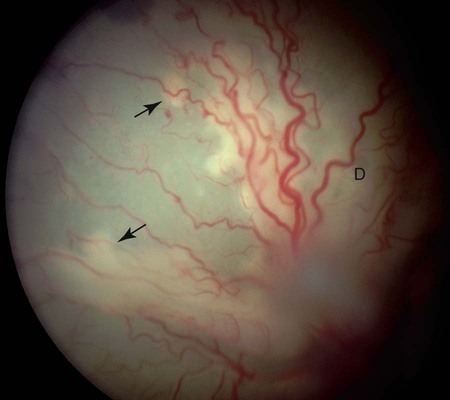
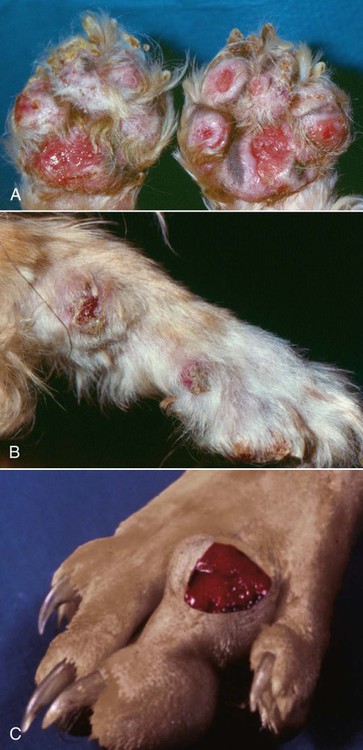
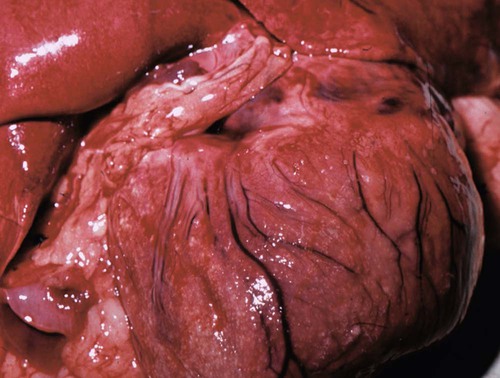
The genus Prototheca (family Chlorellaceae) is made up of unicellular algae that are obligatory saprophytes because they lack chloroplasts capable of photosynthesis.37,50 All described protothecae are morphologically similar, with individual cells being ovoid to oblong in tissue sections or spherical in laboratory cultures and body fluids, and having granular, basophilic cytoplasm and a thick, hyaline cell wall. Reproduction occurs via asexual endosporulation under appropriate environmental conditions with eventual rupture and release of between 2 and 20 daughter cells that grow and in turn again endosporulate. Organism diameter may range from 1.5 µm to 30 µm, with variations in size influenced by species, time since sporulation, and environmental or culture conditions.37,50 Prototheca spp. are ubiquitous and can be routinely isolated from plant surfaces, soil, or water, may occur as transient flora within animal and human gastrointestinal (GI) tracts, and are occasional asymptomatic colonizers of human skin and nail beds.50 However, because of their saprophytic nature, these algae preferentially occupy ecologic niches that offer a continuous source of partially digested or decomposing organic matter in wet environments, that is, raw or treated sewage, cattle or swine manure, and tree slime flux. Historically only three species of Prototheca were recognized: Prototheca stagnora, Prototheca wickerhamii, and Prototheca zopfii. However, results of sequencing ribosomal RNA from several human and animal isolates indicate that previously described biotypes, or “variants,” of P. zopfii should perhaps be considered distinct species. In addition to these genetic differences, they may also occupy distinct environmental niches. P. zopfii biotype 1 is naturally found in bovine liquid manure, whereas most cases of bovine mastitis are due to P. zopfii biotype 2.43,61 P. zopfii biotype 3, which is typically isolated from swine manure rather than bovine manure, has been renamed Prototheca blaschkeae.61 Finally, provisional strain differences in P. wickerhamii have been detected, and a novel Prototheca (Prototheca cutis sp. nov.) has been tentatively identified based on ribosomal RNA sequencing of a single human isolate.64,79 Protothecosis is an uncommon, sporadic disease in people, dogs, cats, and nondomesticated mammals, with human and canine infections reported worldwide. Prototheca spp. infections in dogs occur more frequently in warm, humid regions such as the Gulf Coast of the United States, and similarly, most reported infected people reside in the southern United States, Japan, and southern European countries bordering the Mediterranean.35,37,50,71 No seasonal distribution of disease has been reported in dogs or people. Although protothecosis is recognized with sufficient frequency that individual reports of disease are not routinely published, the relative prevalence of disease is still considered low: by the year 2000, only 108 human infections had been reported in the medical literature; by 2006, only 44 canine infections had been reported; and by 2009, only 5 feline infections had been reported.* In dairy cows, however, outbreaks and endemic herd infections are regularly reported. Prototheca spp. infection most commonly causes mastitis in cows, with the difference in epidemiology likely because these algae thrive in their manure, resulting in direct and continued exposure of animals to high concentrations of organisms.51,65 Milking machines have been suggested as a possible fomite; however, the association between protothecal mastitis and the lactation cycle is disputed.32,65 P. stagnora is presumptively nonpathogenic, because no cases of disease have been associated with this species. However, for the remaining Prototheca spp., the frequency and type of disease varies among people, dogs, cats, and cows (Table 67-1). More than 95% of infections in people are due to P. wickerhamii, with only single reports of infection with P. blaschkeae or P. cutis sp. nov.–associated illness, and the remaining ones with nonbiotyped P. zopfii.35,64 In dogs, most (i.e., 75% to 90%) Prototheca infections are due to nonbiotyped P. zopfii, with one report of P. zopfii biotype 2; the remaining reported infections were due to P. wickerhamii.55,59,59 When they have been speciated, all organisms infecting cats have been P. wickerhamii.12,18 Nonbiotyped P. zopfii or P. zopfii genotype 2 have been isolated from the majority of affected cows, with only occasional case reports of P. blaschkeae infection.40,43,43 However, P. blaschkeae was only recognized as a novel species in 2006, and therefore reported cases of human or canine P. zopfii infection before this time must be questioned. Whether or not cats were infected with P. zopfii and, if so, its respective biotype, is unknown. TABLE 67-1 Prototheca spp. Associated with Disease Syndromes in Domestic Animals and People C, Cutaneous; M, mastitis; O, olecranon bursitis; S, systemic. Traumatic inoculation or contamination of open wounds has been associated with cutaneous infections. In people, some infections occurred with infection of preexisting wounds, such as olecranon bursitis, presumptively due to repeated trauma to this region.35,37 Infection of previously recognized open wounds has not been reported in dogs or cats; however, trauma and contamination may be associated with protothecal mastitis in cows.† Because of the ubiquitous nature of Prototheca spp. and common exposure of animals to these organisms, disseminated disease is attributed to immunosuppression. The most frequently identified causes of immunosuppression in people with disseminated protothecosis are local or systemic glucocorticoid administration, neoplasia (particularly hematologic cancers), diabetes mellitus, and acquired immunodeficiency syndrome.35,37,37 Despite these associated comorbid disorders in people, similar associations have not been made in dogs. Rather than exploiting host immunosuppression, Prototheca spp. may directly alter neutrophil function, thus promoting establishment, persistence, or dissemination of infection. P. zopfii increases antioxidant enzyme and hydrogen peroxide production by bovine milk neutrophils, although these changes in function do not alter opsonization or killing of organisms.15 In one dog with systemic protothecosis, both neutrophil chemotaxis and T-lymphocyte stimulation were reduced as compared to healthy control dogs, but whether these were predisposing alterations or were themselves induced by algal infection is unknown.57 During antifungal therapy of dogs with systemic infection, mononuclear cells increase at sites of organism infiltration, but the type and competence of these cells has not been assessed.49 Hormonal or genetic predisposition may also predispose dogs to infection, because females are overrepresented in both Prototheca spp. case series and the literature in general.42,71 In addition, the predominance of collies with systemic or cutaneous protothecosis in case reports from the United States or Europe has suggested a possible breed association in these geographic areas, as has the frequency of disease in boxers and boxer crosses in Australian cases.42,71 However, neither sex nor breed predisposition to protothecosis has been systematically examined via appropriate statistical analyses. Dogs with disseminated Prototheca spp. infection most commonly have clinical signs referable to infection of the distal GI tract, central nervous system (CNS), and eyes. As a result of colitis, severe, intermittent to persistent large bowel diarrhea with mucus, hematochezia, with or without melena are most commonly reported; occasional signs include voiding of sloughed mucosa, weight loss, or vomiting.42,71 Approximately 50% of dogs may have clinical signs attributable to CNS infection with or without concurrent colitis. Neurologic abnormalities, associated with meningoencephalomyelitis, may include seizures, central vestibular disease, altered mentation, blindness, deafness, ataxia, or lateralizing or generalized lower motor neuron deficits.* In most cases, owner perception is that neurologic signs were of acute onset. Acute blindness may be the result of CNS infection or due to severe ocular inflammation. Ocular disease includes: uveitis-associated glaucoma; miosis, and aqueous flare due to leukokoria; buphthalmos; posterior synechiae; chorioretinitis with raised, white, retinal granulomas; and retinal or vitreous hemorrhage (Fig. 67-1).† Less commonly noted clinical signs may reflect infection of other organs, including peripheral lymphadenomegaly, clinical signs of uremia, lameness due to osteomyelitis, and sudden death due to presumptive protothecal myocarditis.44,55,71,79,87 The appearance and occurrence of cutaneous infections are variable in affected dogs (Fig. 67-2, A and B). Nonulcerated, multifocal military to nodular skin lesions are usually observed first.38,73,73 More severely affected dogs may have ulcerated lesions on the trunk, pinnae, scrotum, foot pads, or planum nasale with associated nasal discharge.24,73,73 Skin lesions either may occur as the sole overt clinical sign or may accompany the previously described GI, neurologic, or ophthalmic abnormalities. Protothecosis is very rare in cats, because of either natural resistance to infection or avoidance of environmental niches where algae are typically found. The few published cases have all described clinically healthy adult cats with firm, nonulcerated, cutaneous or subcutaneous masses on the forehead, distal limbs, base of the tail, nose, or pinnae (see Fig. 67-2, C).12,18,18a,19,33 The absence of regional lymphadenomegaly and lack of any clinical signs of systemic disease in these cats suggest that these infections were localized. However, one cat developed new distant nodules several months after excisional biopsy of an original solitary lesion.18 Hematologic and serum biochemical abnormalities in dogs with disseminated protothecosis generally reflect nonspecific inflammation (i.e., neutrophilic leukocytosis and hyperglobulinemia).26,55,55 In animals with kidney or liver involvement, abnormalities may be those typically associated with renal failure or hepatocellular damage.55,60,60 Urinalysis abnormalities may include minimally concentrated urine or active urine sediment; these findings may be due to renal dysfunction and the frequent renal shedding of Prototheca spp. (see Pathologic Findings sections, later).55,71 Cerebrospinal fluid (CSF) from dogs with CNS signs and vitreous fluid from dogs with uveitis may be grossly discolored and have marked increases in both polymorphonuclear and mononuclear leukocytes and protein concentration.13,57,67,81,86 Initial presumptive diagnosis of cutaneous or disseminated Prototheca spp. infection most commonly occurs via cytologic identification of morphologically consistent organisms in colonic biopsy impression smears, lymph node fine-needle aspirates, or skin or colonic biopsies (Fig. 67-3). Although definitive diagnosis requires culture, the concurrent presentation of large intestinal diarrhea with hematochezia and uveitis, along with the unusual cytologic appearance of Prototheca spp., makes cytologic diagnosis relatively reliable. Organisms in cytologic samples stain well with modified Wright or Gram stain with surrounding nonstaining clear cell walls. Dogs with clinical signs of GI disease may have organisms noted in rectal or colonic scrapings, but this appears to be a less reliable method of diagnosis than endoscopic or full-thickness biopsies of the distal intestine. Examination of routine urine sediment or cytospin preparations identifies organisms in greater than 50% of infected dogs.55,71 Organisms have also been noted in vitreous humor samples from dogs with uveitis.41,60,67,86 In dogs with atypical presentations (e.g., acute renal failure), organisms may be first noted in biopsies from affected organs.55 Definitive diagnosis of Prototheca spp. infection requires culture or genetic identification methods. All Prototheca species grow readily on most laboratory media, including blood agar; therefore, even in cases when protothecosis is not suspected, culture of biologic samples such as urine, CSF, or vitreous humor oftentimes results in abundant algal growth.55,71 Prototheca spp. form white to light tan colonies on blood and Sabouraud’s cycloheximide-free dextrose agar at 25° C to 37° C; differences in sugar and alcohol assimilation or antibacterial susceptibility are required for routine species differentiation, whereas ribosomal RNA sequencing is needed to differentiate P. zopfii biotypes.7,37,37 Differentiation of Candida spp. from Prototheca spp. (both of which may be found in urine) using ribostamycin-impregnated disks has been described, but cytologic examination of colonies is sufficient and more rapid.8 The colon and ileum in dogs with systemic protothecosis are usually erythematous, thickened, and friable, with increased mucosal granularity, erosions, nodules, and large amounts of mucus and frank blood oftentimes seen on endoscopic examination.58,75,75 Gross necropsy findings in dogs with disseminated Prototheca spp. infection may be confined to the GI tract, but more typically include pinpoint to several millimeter-diameter white to yellow plaques or nodules on the serosal surfaces of numerous organs, including the heart, liver, spleen, mesenteric lymph nodes, diaphragm, and thyroid glands (Fig. 67-4).* Larger green to white plaques that extend into underlying tissues, particularly the lungs, may also be present.41
Protothecosis and Chlorellosis
Protothecosis
Etiology
Epidemiology
P. blaschkeae
Cows (M, S); people (C)
P. cutis (sp. nov.)
People (C)
P. stagnora
Nonpathogenic
P. wickerhamii
Cats (C); dogs (C, S); people (C, O, S)
P. zopfii (nonbiotyped)
Cows (M, S); dogs (C, S); people (C, O, S)
P. zopfii biotype 1
Nonpathogenic
P. zopfii biotype 2
Cows (M); dogs (S)
Pathogenesis
Clinical Findings
Dogs
Cats
Diagnosis
Clinical Laboratory Findings
Cytologic Examination
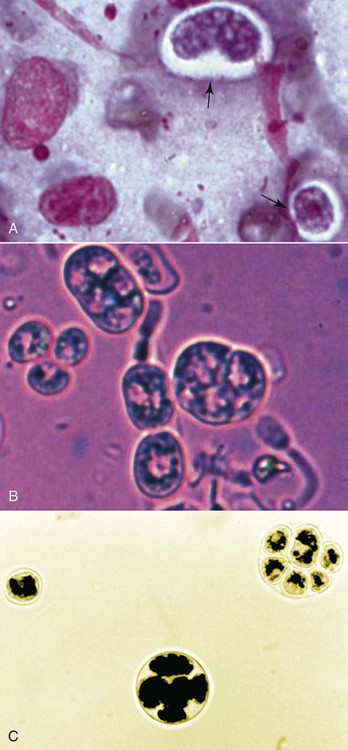
Algal Identification and Isolation
Pathologic Findings
Dogs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Protothecosis and Chlorellosis